Question
Using the data below please make two graphs with Excel (or a similar program).
1. Absorbance (v-axis) vs. A (x-axis) for the absorbance curve of the dye solution. Connect the points with a smooth curve.
2. Absorbance (y-axis) vs. concentration (x-axis) for the Beer's Law data. Draw a best-fit line or use a program such as Excel and do a least-squares fit to determine the best-fit line through the points.
please answer super super fast please please need it now its urgent just creat the graphs
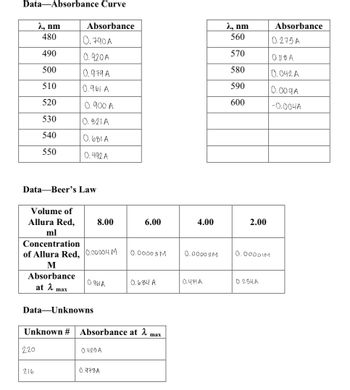
Transcribed Image Text:**Educational Website Transcription**
### Data — Absorbance Curve
#### Table 1: Absorbance Measurements
| λ (nm) | Absorbance |
|--------|------------|
| 480 | 0.790 A |
| 490 | 0.920 A |
| 500 | 0.979 A |
| 510 | 0.961 A |
| 520 | 0.900 A |
| 530 | 0.621 A |
| 540 | 0.604 A |
| 550 | 0.492 A |
| λ (nm) | Absorbance |
|--------|------------|
| 560 | 0.275 A |
| 570 | 0.118 A |
| 580 | 0.042 A |
| 590 | 0.009 A |
| 600 | -0.004 A |
### Data — Beer’s Law
#### Table 2: Beer’s Law Data
| Volume of Allura Red (ml) | Concentration of Allura Red (M) | Absorbance at λₘₐₓ |
|---------------------------|---------------------------------|--------------------|
| 8.00 | 0.00004 M | 0.981 A |
| 6.00 | 0.00003 M | 0.684 A |
| 4.00 | 0.00002 M | 0.474 A |
| 2.00 | 0.00001 M | 0.254 A |
### Data — Unknowns
#### Table 3: Unknown Samples
| Unknown # | Absorbance at λₘₐₓ |
|-----------|---------------------|
| 220 | 0.426 A |
| 216 | 0.973 A |
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 5 images

Knowledge Booster
Similar questions
- The following describes similarities between true solutions and colloidal dispersions 1. Filtering is not an effective method of particle separation/isolation 2. Particles deflect light 3. Particles cannot be observed without magnification. arrow_forwardTASK 1: DISCUSSION 1 Discuss diffusion mechanism below. Explain in terms of mechanism process and how it is important in material industries. Explain in Two (2) pages with at least 10 journal references. Figure 1: Vacancy diffusion shows motion of the host atom to vacant space End of documentarrow_forwardA typical daily shower uses 25 litres of water at 42 degrees celsius. If the incoming mains water is at 8 degrees celsius, how much energy in kWh (to two decimal places), is used? Note: SHC of water = 4186 J/kgK Answer: ge Next page VIOUS ACTIVITY rld Oil Production_Curve Fitting 09 10 20 NEXT ACTIVITY >> How PV cell produces electricity Jump to... School Links Staff Links nt Links Learnonline Course Request 2020-21 (Staff Only) School of Business School of Engineering Schoolof Science esults Learnonline Archivearrow_forward
- Quiz 5 Calculate the plane density of (l1o) plane at BCC Cystal structurearrow_forwardDirections: Read and analyze each situation. Answer the questions that follow 1. Two pans of water are on different burners of a stove. One pan of water is boilingvigorously, while the other is boiling gently. What can be said about the temperature ofthe water in the two pans? 2. A large container of water and a small one, are at the same temperature. What canbe said about the relative vapor pressures of the water in the two containers?arrow_forwardWith the data shown below, please answer the following: A. Which of the two inhibitors is more efficient at high substrate concentrations? At low substrate concentrations? Explain your reasoning. Determine the binding constant (KI) for inhibitors A and B. Show the calculations.arrow_forward
- Please complete the below problems: 1. An aluminum aloy 2024-T651 (see Table 6.1 in your book) panel is sub- jected to biaxial loading as shown in below. Assume that o = 300 MPa and oz can be either tension or compression. Find the maximum values of lo2] in tension and compression that the panel can withstand before yielding according to von Mises yield criterion. Figure 1: Biaxial loading.arrow_forwardFill in the blank with correct answers: ohms f(Hz) The graph of reactance against frequency, shown in the picture is for a Average kinetic energy of the gas molecules is O a. Independent of absolute temperature O b. Directly proportional to the 4th power of absolute temperature Directly proportional to absolute temperature O d. Inversely proportional to absolute temperaturearrow_forwardThe conversion of cyclopropane in the gas phase is a first-order reaction with a rate constant of 6.7 x 10-4 s1at 500°C. If the initial concentration is O.25 M, what is the concentration after 8.8 mins? Assume first order reaction. 1 Add filearrow_forward
arrow_back_ios
arrow_forward_ios