
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
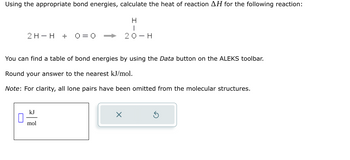
Transcribed Image Text:Using the appropriate bond energies, calculate the heat of reaction AH for the following reaction:
H
I
20-H
2 H- H + 0=0
You can find a table of bond energies by using the Data button on the ALEKS toolbar.
Round your answer to the nearest kJ/mol.
Note: For clarity, all lone pairs have been omitted from the molecular structures.
0
kJ
mol
X
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- need help with this chemistry reviewarrow_forwardI need help calculating the enthalpy of this reaction please.arrow_forwardUsing the bond energies below (kJ/mol), calculate the estimated ΔH of the reaction pictured below the table. Express your answer in kJ/mol. Screen reader note: "=" is read as a double bond. "identical to" is read as a triple bond. All other bonds are single bonds. End of note. H-H 436 H-C 414 H-N 389 H-O 464 H-S 368 H-F 565 H-Cl 431 H-Br 364 H-I 297 C-C 347 C=C 611 C≡C 837 C-N 305 C=N 615 C≡N 891 C-O 360. C=O 736 C-Cl 339 N-N 163 N=N 418 N≡N 946 N-O 222 N=O 590. O-O 142 O=O 498 C-Br 293 - F-F 159 Cl-Cl 243 Br-Br 193 I-I 151 - - - - - 2 H-O-O-H ----> 2 H-O-H + O=O (OR 2 H single bond O single bond O single bond H right arrow 2 H single bond O single bond H + O double bond Oarrow_forward
- Use table to calculate Delta H in kJ for the synthesis of hydrazine from ammonia 2NH3 (g) + Cl2 (g) ——> N2H4 (g) + 2HCl (g)arrow_forwardORT SHEET Heat of Neutralization EXPERIMENT elemsboomfchg lom 12 A. Heat Capacity of Calorimeter 1. Temp. of calorimeter and water before mixing 2. Temp. of warm water °C 22.0 39,0 30.3 3. Maximum temp. determined from your curve °C 4. Heat lost by warm water (temp decrease x °C 50.0 g x 4.184 J/K-g) = 02), 5. Heat gained by cooler water (temp. increase x 50.0 g x 4.184 J/K-g) = 30,3 22.0)x 13626J s0.0gmpi S0.0gy 6. Heat gained by the calorimeter [(4) – (5)] = 7. Heat capacity of calorimeter: heat gained by the calorimeter temperature increase J/K 3. Heat of Neutralization of HCl-NaOH 22.2 22.2. °C . Temp. of calorimeter and NaOH Temp. of HCI AT determined from your curve after adding HC1 °C to the NaOH Heat gained by solution (temperature increase x ON 100 g x 4.184 J/K-g) = 9977.8J %3D Heat gained by calorimeter (temperature increase x heat capacity of calorimeter) = J %3D Total joules released by reaction [(3) + (4)] = Tight O 2018 Pearson Education, Inc.arrow_forwardgrade 12 chem: Briefly describe four DIFFERENT ways to determine the enthalpy change (ΔH) of a reactionarrow_forward
- Calculate the enthalpy of the reaction below (AHrxn, in kJ) using the bond energies provided. H₂(g) + CH3CH₂C(=O)H → CH3CH₂CH₂OH Single Bond H C N O Multiple Bonds H 432 411 346 386 459 358 C **All values in kJ/mol** 305 167 201 C=C C=C 835 N O 602 C=O C=O C=N C=N 887 615 O=O N=N 142 799 1072 494 942arrow_forwardQuestion 7 of 8 O Macmillan Learning Alkane halogenation is a two-step reaction, as shown in the image. Using the table of bond dissociation energies, calculate the enthalpy of each step and the enthalpy of the overall reaction. Bond Dissociation Energies (for A-B Bond broken AH (kJ/mol) Bond broken AH (kJ/mol) Bond broken AĦ (kJ/mol) H-H 436 400 366 292 193 Step 1 H3C- Step 2 H3C- CH3 CH3 CH3 CH3 Overall reaction H + + Br₂ CH3 I H3C- CH3 -H + Br₂ (CH₂)₂C-H (CH3)3C-Br H3C H3C A H3C- ■ CH3 CH3 + CH3 CH 3 + -Br CH3 F CH3 ■ B) + HBr + Br: -Br + HBr H-Br Br-Br ΔΗ = AH = AH = kJ/mol kJ/mol kJ/molarrow_forwardUsing the table of bond enthalpies in your textbook, predict A-H for the following reaction. Express your answer in kJ/mol. H₂C=CH₂ + Cl₂ → CI-CH₂-CH₂-CI Answer:arrow_forward
- Using the following bond enthalpies H-H F-F H-F 436 kJ/mol 157 kJ/mol 568 kJ/mol calculate AH for the reaction: H-H(g) + F-F(g) O-543 kJ 543 kJ 25.0 kJ O-1090 kJ O-25.0 kJ 2 H-F(g)arrow_forwardCalculate the heat of reaction AH for the following reaction: 2 HCl(g) + Br,(g)→2 HBr(g) + Cl,(g) You can find a table of bond energies by using the Data button on the ALEKS toolbar. Round your answer to the nearest kJ/mol. kJ ? molarrow_forwardUsing the appropriate bond energies, calculate the heat of reaction AH for the following reaction: 1 61010 mol CI-C-CI+ H-H You can find a table of bond energies by using the Data button on the ALEKS toolbar. Round your answer to the nearest kJ/mol. Note: For clarity, all lone pairs have been omitted from the molecular structures. c-c-c + H-CI -4-0 H X ?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY