
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
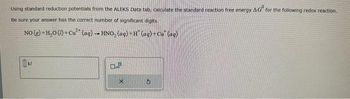
Transcribed Image Text:Using standard reduction potentials from the ALEKS Data tab, calculate the standard reaction free energy AG for the following redox reaction.
Be sure your answer has the correct number of significant digits.
NO(g) + H₂0 (1) + Cu (aq) → HNO₂ (aq) + H¨ (aq) + Cu (aq)
0.P
X
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- ▼ Part A Complete and balance the half-reaction in basic solution. O2(g) → OH(aq) Express your answer as a chemical equation including phases. Submit ΑΣΦ Part B → A chemical reaction does not occur for this question. $SSA Request Answer ? Complete and balance the half-reaction in basic solution. Mn²+ (aq) → MnO2 (s) Express your answer as a chemical equation including phases.arrow_forwardWhich of the following is an oxidation-reduction reaction? OHCl(aq) + LiOH(aq) → LiCl(aq) + H₂O(1) Nal(aq) + AgNO3(aq) → Agl(s) + NaNO3(aq) Pb(C₂H3O2)2(aq) + 2 NaCl(aq) → PbCl2(s) + 2 NaC₂H3O₂(aq) Mg(s) + 2 HCl(aq) → MgCl₂(aq) + H₂(g) All of these are oxidation-reduction reactions.arrow_forwardHow do we solve this redox reaction?arrow_forward
- From the Lewis structures of the species given, pick all of those in which the central atom obeys the octet rule. OH-N-CI: U U :: | H :F: I. -Br T :F: H-B-H :F: €–8–6 *F: None of the Abovearrow_forwardBalance the redox reaction occurring in basic solution. Cl2(g)+Mn2+(aq)→MnO2(s)+Cl−(aq)Cl2(g)+Mn2+(aq)→MnO2(s)+Cl−(aq) Express your answer as a chemical equation. Identify all of the phases in your answerarrow_forwardComplete the tables below for each : a) 4 Al (s) + 3 O2 (g) → 2 Al2O3 b) Fe (s) + SnCl2 (aq) → FeCl2 (aq) + Sn (s) Element, initial oxidation number -> Final oxidation number, ∆ e-, Oxidized or reduced, Oxidizing agent or reducing agentarrow_forward
- 2. Create a reduction half-reaction table based on the information collected below. Identify the strongest Oxidizing Agent and Reducing Agent in your table. 2 Ce(s) + 3 Ni²+ (aq) → 2 Ce³+ (aq) + 3 Ni(s) Pt(s) + 2 H+ (aq) → no reaction Ni(s) + 2 H*(aq) → Ni²+ (aq) +H2(g) 3 Sr(s) + 2 Ce³+ (aq) →3 Sr³+ (aq) + 2 Ce(s)arrow_forwardBalance the following redox reaction in the gas phase. Note that this reaction cannot be balanced with the half-reaction method and must be done by inspection. In the gas phase, H2 O (1) is used to balance oxygen atoms and H3 O+ and OH are not available for balancing purposes. ONLY integers should be used in your answer. NH3 (aq) + O2 (g) –→ NO2 (g) Enter your chemical notation herearrow_forwardPart A Consider the following unbalanced redox reaction. MnO, (aq) + Zn (s) → Mn²+(ag) + Zn²+(ag) Balance the equation: a MnOq (ag) + BH (ag) + y Zn (s) → 8 Mn2 (ag) + € H2O(1) + K Zn² (ag) Give your answer as an ordered set of numbers a, B, y, .. Use the least possible integers for the coefficients. Templates Symbols uado redo reset keyboard shortcuts help, α, β, γ, δ, ε, κ Submit Request Answer Part B Determine the volume of a 0.560 mol L-'KMN04 solution required to completely react with 2.65 g of Zn. Templates Symbols undo redo reset keyboard shortcuts help, V = mLarrow_forward
- When the following reaction is correctly balanced, how many electrons are transferred? Al3+ (aq) + Ag (s) → Al (s) + Ag+ (aq)arrow_forwardWrite a balanced net ionic equation for a redox reaction that results in the formation of potassium chloride. (Assume that potassium chloride is not a starting material, and assume the reaction takes place in aqueous solution.) X, Не (aq), 2K(ag)+Cl, (aq) KCI(aq)arrow_forwardUsing standard reduction potentials from the ALEKS Deta tab, calculate the standard reaction free energy AG for the following redox reaction. Round your answer to 3 significant digits. Cu (aq) + HNO₂ (aq) + H* (aq) → Cu² (aq) + NO(g) + H₂O (1) Xarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY