
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
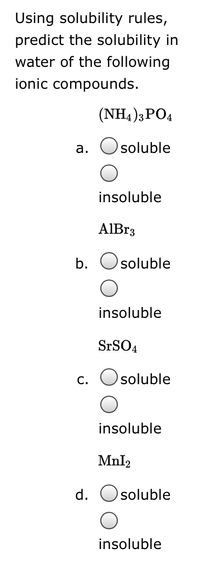
Transcribed Image Text:Using solubility rules,
predict the solubility in
water of the following
ionic compounds.
(NH4 )3PO4
а.
soluble
insoluble
AIB33
b.
soluble
insoluble
SrSO4
c. Osoluble
insoluble
Mnl2
d.
soluble
insoluble
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What is the name of the process by which a solute dissolves in a solvent? OA. Precipitation B. Dissociation C. Ionization OD. Solvationarrow_forwardA solution is prepared by adding 150.0 mL of a 0.0100 M Mg(NO3)2 solution to 250.0 mL of a 0.100 M NaF solution. Will anything precipitate from this solution and, if so, what is the precipitate? a. No, nothing will precipitate. b. Yes, MgF 2 c. There is not enough information to answer this question. d. Yes, Mg(NO 3 ) 2 e. Yes, NaNO 3arrow_forwardWill adding water to SbCl + H20 <----> SbOCl + 2HCl form more or less precipitate? Will adding hydrochloric acid to SbCl + H20 <----> SbOCl + 2HCl form more or less precipitate?arrow_forward
- KSP for Copper Sulfide is: 7.9 × 10-37 KSP for Copper Hydroxide is: 1.6 × 10-19arrow_forwardA solution contains 1.22x10-2 M lead nitrate and 1.05x10-² M silver acetate. Solid sodium chloride is added slowly to this mixture. What is the concentration of silver ion when lead ion begins to precipitate? [Ag*]= Submit Answer Retry Entire Group 7 more group attempts remainingarrow_forwardThe Solubility Product Constant for nickel(II) hydroxide is 2.8 x 10 ^-16 The maximum amount of nickel(II) hydroxide that will dissolve in a 0.130 M nickel(II) nitrate solution is _______M.arrow_forward
- A solution contains 9.47x10^-3 M nickel (II) acetate and 1.27x10^-2 M zinc nitrate. Solid potassium hudroxide is added slowly to this mixture. what is the concentration of zinc ion when nickel ion begins to precipitate? [Zn^2+]=_________Marrow_forwardHow do i solve for the precipitates?arrow_forward1. Using the solubility chart, explain whether or not this double replacement reaction will occur. FeSO4 + NaCl -> Na2SO4 + FeCl2 2. In order for a double replacement reaction with precipitate to occur, what must be true. Select all that apply The ion doing the replacing must be more reactice than the ion it replaces Both reactants must be soluble in water Both products must be soluble in water One of the reactants must be insolube in water One of the products must be insoluble in waterarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY