
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
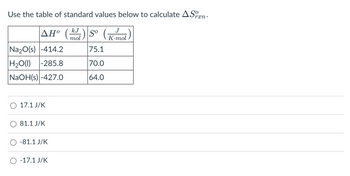
Transcribed Image Text:Use the table of standard values below to calculate ASxn.
rxn•
ΔΗ°
Na₂O(s) -414.2
H₂O(l) -285.8
NaOH(s) -427.0
O 17.1 J/K
81.1 J/K
-81.1 J/K
O -17.1 J/K
kJ
So
mol
75.1
70.0
64.0
J
K.mol
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- options for all: 1. Reactants. 2. Products.arrow_forwardIdentify the reactions that are exothermic. ) Multiple answers: Multiple answers are accepted for this question Select one or more answers and submit. For keyboard navigation... SHOW MORE V C(graphite)C(diamond) AH° = 2 kJ b. CaCO3 (s) CaO(s) + CO2(g) AH° = 178 kJ CuO(s) + H2 (g) → Cu(s) + H2 0(g) AH° = -85 kJ 2H2 (g) + 02(g) - 2H20(g) AH = -484 kJ 3H2 (g) + N2(g) 2NH3 (g) AH - 92 kJ Unansweredarrow_forwardFor a particular reaction at 150.5 °C, AG = –392.62 kJ/mol, and AS = 692.39 J/(mol · K). Calculate AG for this reaction at -0.4 °C. - AG = kJ/mol 80 DII DD F3 F4 F7 FB F9 F10 F12 %24 & 4. 5 6 7 8 Y U H K F.arrow_forward
- 1. A reaction with AH° = -64.0 kJ/ mole has K = 1.00 X 108 at 25.0 °C. What would be the value of K at 0.00 °C? 2. A reaction with AH° = 618.0 kJ/mole has K = 1.00 X 1060 at 25.0 °C. What would be the value of K at 500.0 °C?arrow_forwardCircle any of the following that would be classified as a heterocyclic amine. NH₂ NH₂ NH₂arrow_forwardConsider the following reaction: H2(g)+I2(g)-->2HI(g) Given the following data: H2: delta H*= 0 kJ/mol; S*= 131 J/mol K I2: delta H*= 0 kJ/mol; S*= 104 J/mol K HI: delta H*= 25 kJ/mol; S*= 206 J/mol K Determine the delta G* in kJ for this reaction at 298K.arrow_forward
- Calculate G for the reaction of iron (III) oxide with carbon monoxide to give elemental iron and carbon dioxide. í G₁ (kJ/mol) Fe(s) 0 CO₂(g) -394.4 Fe₂O3(s) -741.0 CO(g) -137.3arrow_forwardThe reusable booster rockets of the space shuttle use a mixture of aluminum and ammonium perchlorate as fuel. A possible reaction is 3A1(s) + 3NH, Cl0,(8) → Al, O, (s) + AICI; (s) + 3NO(9) + 6H2O(g) Calculate AH° for this reaction. Substance and State AH ° (kJ/mol) Al(s) Al2 O3 (s) AICI3 (s) H2O(g) NO(g) NH, CIO4 (s) -1676 -704 -242 90. -295 AH° = kJarrow_forwardConsider the reaction. 2 Fe,O, 4 Fe + 30, AHn = +824.2 kJ The formation of 84.0 g of Fe results in the absorption of 3.10 x 102 kJ of heat. the absorption of 17300 kJ heat. the absorption of 1240 kJ of heat. the release of 1240 kJ of heat. the release of 3.10 x 10² kJ of heat. the release of 17300 kJ of heat. G Search or type URLarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY