
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
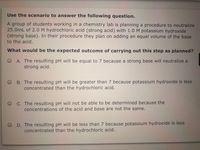
Transcribed Image Text:Use the scenario to answer the following question.
A group of students working in a chemistry lab is planning a procedure to neutralize
25.0mL of 2.0 M hydrochloric acid (strong acid) with 1.0 M potassium hydroxide
(strong base). In their procedure they plan on adding an equal volume of the base
to the acid.
What would be the expected outcome of carrying out this step as planned?
O A. The resulting pH will be equal to 7 because a strong base will neutralize a
strong acid.
B. The resulting pH will be greater than 7 because potassium hydroxide is less
concentrated than the hydrochloric acid.
C. The resulting pH will not be able to be determined because the
concentrations of the acid and base are not the same.
D. The resulting pH will be less than 7 because potassium hydroxide is less
concentrated than the hydrochloric acid.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A chemist dissolves 596. mg of pure barium hydroxide in enough water to make up 140, ml. of solution. Calculate the pH of the solution. (The temperature of the solution is 25 °C.) Round your answer to 3 significant decimal places. 0 X 2arrow_forwardWhat volume of a concentrated HCl solution, which is 36.0% HCl by mass and has a density of 1.179 g/mL, should be used to make 5.10 L of an HCl solution with a pH of 1.60? Express your answer to two significant figures and include the appropriate units. HẢ ? V = Value Unitsarrow_forwardA chemist must prepare 800.0 mL of sodium hydroxide solution with a pH of 13.70 at 25 °C. She will do this in three steps: • Fill a 800.0 mL volumetric flask about halfway with distilled water. Weigh out a small amount of solid sodium hydroxide and add it to the flask. • Fill the flask to the mark with distilled water. Calculate the mass of sodium hydroxide that the chemist must weigh out in the second step. Round your answer to 2 significant digits. x10arrow_forward
- Finding pH An unknown substance has a hydrogen ion concentration of 3H 4 3.1 108M. Find the pH and classify the substance as acidic or basicarrow_forwardA student is provided with a stock solution of NaOH with 0.1 M concentration. A 25.0 mL of stock solution of NaOH is diluted with water to prepare 500.0 mL NaOH a.Calculate the molarity of the final diluted b.Calculate the hydronium ion concentration, [H3O+] in the final c.Calculate the pH of the diluted NaOH solution d.Is the final diluted solution acidic, basic or neutral?arrow_forwardA chemist must prepare 600.0 mL of sodium hydroxide solution with a pH of 13.40 at 25 °C. She will do this in three steps: • Fill a 600.0 mL volumetric flask about halfway with distilled water. Weigh out a small amount of solid sodium hydroxide and add it to the flask. • Fill the flask to the mark with distilled water. Calculate the mass of sodium hydroxide that the chemist must weigh out in the second step. Round your answer to 2 significant digits. x10 ?arrow_forward
- A chemist dissolves 890. mg of pure barium hydroxide in enough water to make up 110. mL of solution. Calculate the pH of the solution. (The temperature of the solution is 25 °C.) Round your answer to 3 significant decimal places. x10arrow_forwardA chemist must prepare 850.0 mL of sodium hydroxide solution with a pH of 12.60 at 25 °C. He will do this in three steps: • Fill a 850.0 mL volumetric flask about halfway with distilled water. • Weigh out a small amount of solid sodium hydroxide and add it to the flask. • Fill the flask to the mark with distilled water. Calculate the mass of sodium hydroxide that the chemist must weigh out in the second step. Round your answer to 2 significant digits. Ox10arrow_forwardConsider the following data on some weak acids and weak bases: name acetic acid hydrocyanic acid acid solution 0.1 M KCH3CO₂ 0.1 M NaBr 0.1 M NaCN 0.1 M C5H5NHCI Ka formula HCH3CO2 1.8 × 10 HCN 4.9 × 10 Use this data to rank the following solutions in order of increasing pH. In other words, select a '1' next to the solution that will have the lowest pH, a '2' next to the solution that will have the next lowest pH, and so on. X pH - 10 -5 choose one choose one ✓ choose one choose one Ś base K₂ formula C6H5NH₂ 4.3 × 10 -10 -9 1.7×10 ⁹ name aniline pyridine CH-Narrow_forward
- A chemist must prepare 900.0 mL of sodium hydroxide solution with a pH of 13.40 at 25 °C. He will do this in three steps: • Fill a 900.0 mL volumetric flask about halfway with distilled water. • Weigh out a small amount of solid sodium hydroxide and add it to the flask. • Fill the flask to the mark with distilled water. Calculate the mass of sodium hydroxide that the chemist must weigh out in the second step. Round your answer to 2 significant digits. Ox10arrow_forwardA chemist dissolves 522. mg of pure potassium hydroxide in enough water to make up 120. mL of solution. Calculate the pH of the solution. (The temperature of the solution is 25 °C.) Round your answer to 3 significant decimal places. x10 5arrow_forwardA stock solution of hydrochloric acid has a concentration of 0.1200M. It is diluted by transferring 10.00 mL to a 100mL volumetric flask using a volumetric pipette. Based on calculations it is found the pH is 1.92. Using the solution that was diluted, prepare a solution with a pH of 2.912. What volume of the diluted solution should you dilute to 100.00 mL to make this solution?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY