
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Transcribed Image Text:OMO
req
Seiten
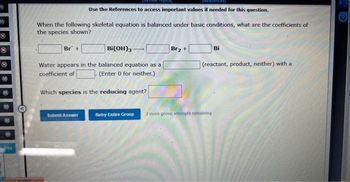
Use the References to access important values if needed for this question.
When the following skeletal equation is balanced under basic conditions, what are the coefficients of
the species shown?
Bi(OH)3
Water appears in the balanced equation as a
coefficient of
(Enter 0 for neither.)
Br +
Which species is the reducing agent?
Submit Answer
Retry Entire Group
Br₂ +
Bi
(reactant, product, neither) with a
2 more group attempts remaining
Transcribed Image Text:q
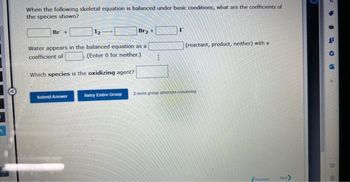
When the following skeletal equation is balanced under basic conditions, what are the coefficients of
the species shown?
Br +
Br₂ +
Water appears in the balanced equation as a
coefficient of
Which species is the oxidizing agent?
Submit Answer
(Enter 0 for neither.). I
(reactant, product, neither) with a
Retry Entire Group 2 more group attempts remaining
Previou Next
9
→
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- When the following equation is balanced properly under acidic conditions, what are the coefficients of the species shown?Mn + Hg2+Mn2+ + HgWater appears in the balanced equation as a fill in the blank 5 (reactant, product, neither) with a coefficient of . (Enter 0 for neither.)How many electrons are transferred in this reaction?arrow_forwardIn the reaction: 2NO02 + Cu2+ + 2H20 --> 2HNO3 + Cu + 2H* Which of the following statements is correct? HNO3 is the reducing agent, and Cu is the oxidizing agent HNO3 is the reducing agent, and H* is the oxidizing agent Cu is the reducing agent, and HNO3 is the oxidizing agent O Cu is the reducing agent, and H* is the oxidizing agent H* is the reducing agent, and Cu is the oxidizing agentarrow_forwardWhen the following equation is balanced properly under acidic conditions, what are the coefficients of the species shown? Pb2+ + Ni- Pь + Ni2+ Water appears in the balanced equation as a (reactant, product, neither) with a coefficient of (Enter 0 for neither.) Which element is oxidized?arrow_forward
- When the following half reaction is balanced under acidic conditions, what are the coefficients of the species shown? |NO3 + |н". HNO, + H2O In the above half reaction, the oxidation state of nitrogen changes from to Submit Answer Retry Entire Group 8 more group attempts remainingarrow_forwardWhen the following equation is balanced properly under basic conditions, what are the coefficients of the species shown? Cr(OH)3 + F2 F- + CrO42-Water appears in the balanced equation as a fill in the blank _____ (reactant, product, neither) with a coefficient of ___. (Enter 0 for neither.)How many electrons are transferred in this reaction? ____arrow_forwardFor the following unbalanced oxidation-reduction reaction, circle the one oxidizing agent and underline the one reducing agent MnO, + C0, → Mn + COz Cacodyl has a mass composition of 22.88% C. 5.76 % H, and 71.36 % A. What is the empirical formala of cacodyl? How many protons, neutrons and electrons are present in protons neutrons electrons 12 10 7 a) b) 79arrow_forward
- When the following skeletal equation is balanced under acidic conditions, what are the coefficients of the species shown?Mn2+ + SO42-MnO4- + SO2Water appears in the balanced equation as a fill in the blank 5 (reactant, product, neither) with a coefficient of . (Enter 0 for neither.) Which species is the oxidizing agent?arrow_forwardPlease don't provide handwriting solutionarrow_forwardFor a particular redox reaction, NO is oxidized to NO, and Cu²+ is reduced to Cu+. Complete and balance the equation for this reaction in basic solution. The phases are optional. balanced reaction: NO + 3Cu²+ + 2H,O NO, + 3Cu+ + 4H+ Incorrectarrow_forward
- When the following skeletal equation is balanced under acidic conditions, what are the coefficients of the species shown? Ni2+ + Mn -1 Ni + 3 Mn2+ Water appears in the balanced equation as a reactant (reactant, product, neither) with a coefficient of 1 (Enter O for neither.) Which species is the reducing agent? NI"arrow_forwardWhen the following equation is balanced properly under basic conditions, what are the coefficients of the species shown? cl' + Cl2 + s2- S Water appears in the balanced equation as a neither (reactant, product, neither) with a coefficient of 0 (Enter O for neither.) How many electrons are transferred in this reaction? 2arrow_forwardWhen the following equation is balanced properly under acidic conditions, what are the coefficients of the species shown? CIO3 Water appears in the balanced equation as a Co + Which element is reduced? Submit Answer Co2+ [Review Topics] [References] Use the References to access important values if needed for this question. + CI (reactant, product, neither) with a coefficient of Retry Entire Group 6 more group attempts remaining (Enter 0 for neither.)arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY