
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
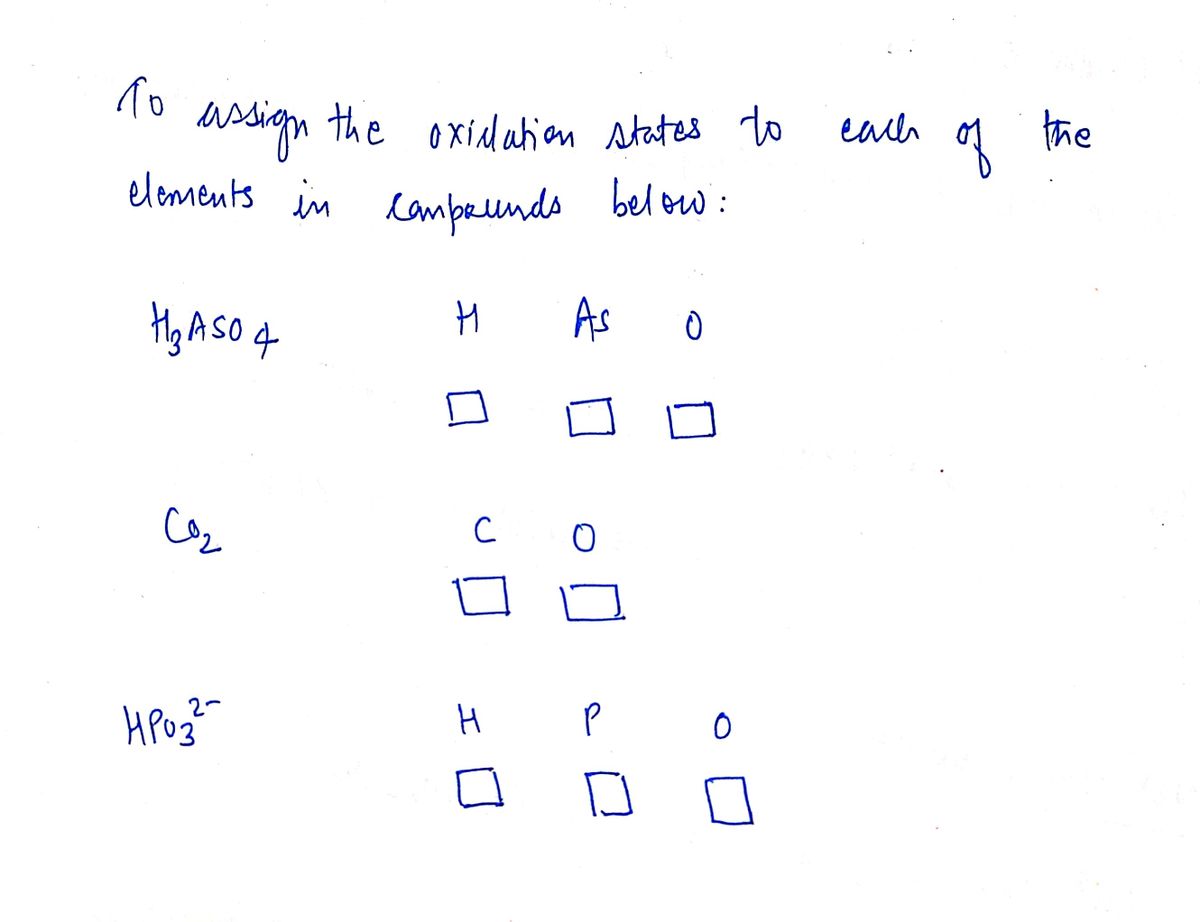
![**Assigning Oxidation Numbers: Practice Exercise**
Use the rules (in order) to assign oxidation numbers to each of the elements in the compounds listed below.
1. **Arsenic Acid (H₃AsO₄)**
- Elements: H (Hydrogen), As (Arsenic), O (Oxygen)
- Input fields for assigning oxidation numbers: [Dropdown for H], [Dropdown for As], [Dropdown for O]
2. **Carbon Dioxide (CO₂)**
- Elements: C (Carbon), O (Oxygen)
- Input fields for assigning oxidation numbers: [Dropdown for C], [Dropdown for O]
3. **Hydrogen Phosphite Ion (HPO₃²⁻)**
- Elements: H (Hydrogen), P (Phosphorus), O (Oxygen)
- Input fields for assigning oxidation numbers: [Dropdown for H], [Dropdown for P], [Dropdown for O]
**Buttons:**
- "Submit Answer" to check your answers.
- "Retry Entire Group" to retry the exercise.
Note: You have 9 more group attempts remaining.
**Instructions:**
Use the provided dropdown menus to assign oxidation numbers to the respective elements in each compound, adhering to the standard rules for determining oxidation states.
---
This exercise encourages understanding of basic chemical principles and helps in practicing the assignment of oxidation numbers, crucial for balancing equations and redox reactions.](https://content.bartleby.com/qna-images/question/32bd268b-b979-4081-864c-934854ccb129/90445fb8-0eb4-4c89-a62d-cef4bb5c4786/999gsdq_thumbnail.jpeg)
Transcribed Image Text:**Assigning Oxidation Numbers: Practice Exercise**
Use the rules (in order) to assign oxidation numbers to each of the elements in the compounds listed below.
1. **Arsenic Acid (H₃AsO₄)**
- Elements: H (Hydrogen), As (Arsenic), O (Oxygen)
- Input fields for assigning oxidation numbers: [Dropdown for H], [Dropdown for As], [Dropdown for O]
2. **Carbon Dioxide (CO₂)**
- Elements: C (Carbon), O (Oxygen)
- Input fields for assigning oxidation numbers: [Dropdown for C], [Dropdown for O]
3. **Hydrogen Phosphite Ion (HPO₃²⁻)**
- Elements: H (Hydrogen), P (Phosphorus), O (Oxygen)
- Input fields for assigning oxidation numbers: [Dropdown for H], [Dropdown for P], [Dropdown for O]
**Buttons:**
- "Submit Answer" to check your answers.
- "Retry Entire Group" to retry the exercise.
Note: You have 9 more group attempts remaining.
**Instructions:**
Use the provided dropdown menus to assign oxidation numbers to the respective elements in each compound, adhering to the standard rules for determining oxidation states.
---
This exercise encourages understanding of basic chemical principles and helps in practicing the assignment of oxidation numbers, crucial for balancing equations and redox reactions.
Expert Solution
arrow_forward
Step 1
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Write a net ionic equation for the reaction that occurs when aqueous solutions of nitric acid and ammonia are combined. +arrow_forwardDoes a reaction occur when aqueous solutions of chromium(III) nitrate and sodium sulfate are combined? Oyes O no If a reaction does occur, write the net ionic equation. Use the solubility rules provided in the OWL Preparation Page to determine the solubility of compounds. Be sure to specify states such as (aq) or (s). If a box is not needed leave it blank. + -arrow_forwardDetermine the oxidation state for each of the elements below. The oxidation state of carbon dioxide in carbon is CO2 water The oxidation state of охуgen in is H2O The oxidation state of arsenious acid is arsenic in H3ASO3arrow_forward
- What is(are) the spectator ion(s) for the following reaction?Ag2NO2 + HI → No spectator ion. All ions are spectator ions, no reaction. NO2‾ H+ and NO2‾arrow_forward80°F Sunny The oxidation number of an element can vary. O True Search F8 6725 F9 O False - « Οι F10arrow_forwardGive the net ionic equation for the reaction (if any) that occurs when aqueous solutions of H2SO4 and KOH are mixed.arrow_forward
- Be sure to specify states such as (aq) or (s). If a box is not needed leave it blank. When aqueous solutions of sodium phosphate and calcium iodide are combined, solid calcium phosphate and a solution of sodium iodide are formed. The net ionic equation for this reaction is: + +arrow_forwardHelp pleasearrow_forwardDetermine net ionic equations, if any, occuring when aqueous solutions of the following reactants are mixed. Select "True" or "False" to indicate whether or not the stated reaction (or "no reaction") correctly corresponds to the expected observation in each case. Magnesium chloride and sodium hydroxide; No reaction occurs. Sodium bromide and hydrochloric acid; No reaction occurs. Copper(II) sulfate and ammonium carbonate; No reaction occurs. Nickel(II) chloride and lead(II) nitrate; No reaction occurs. Sodium phosphate and potassium nitrate; 2Na+ (aq) + NO2- 3 (aq) ------> Na2NO3(s)arrow_forward
- What is the reaction mechanism for the major product? Please draw.arrow_forwardSpecify what ions are present in solution upon dissolvingeach of the following substances in water: (a) FeCl2,(b) HNO3, (c) 1NH422SO4, (d) Ca1OH22arrow_forwardWrite a balanced equation for the double-replacement precipitation reaction described, using the smallest possible integer coefficients. A precipitate forms when aqueous solutions of lead (II) nitrate and sodium hydroxide are combined. Be sure to include states such as (s) or (aq). + +arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY