
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
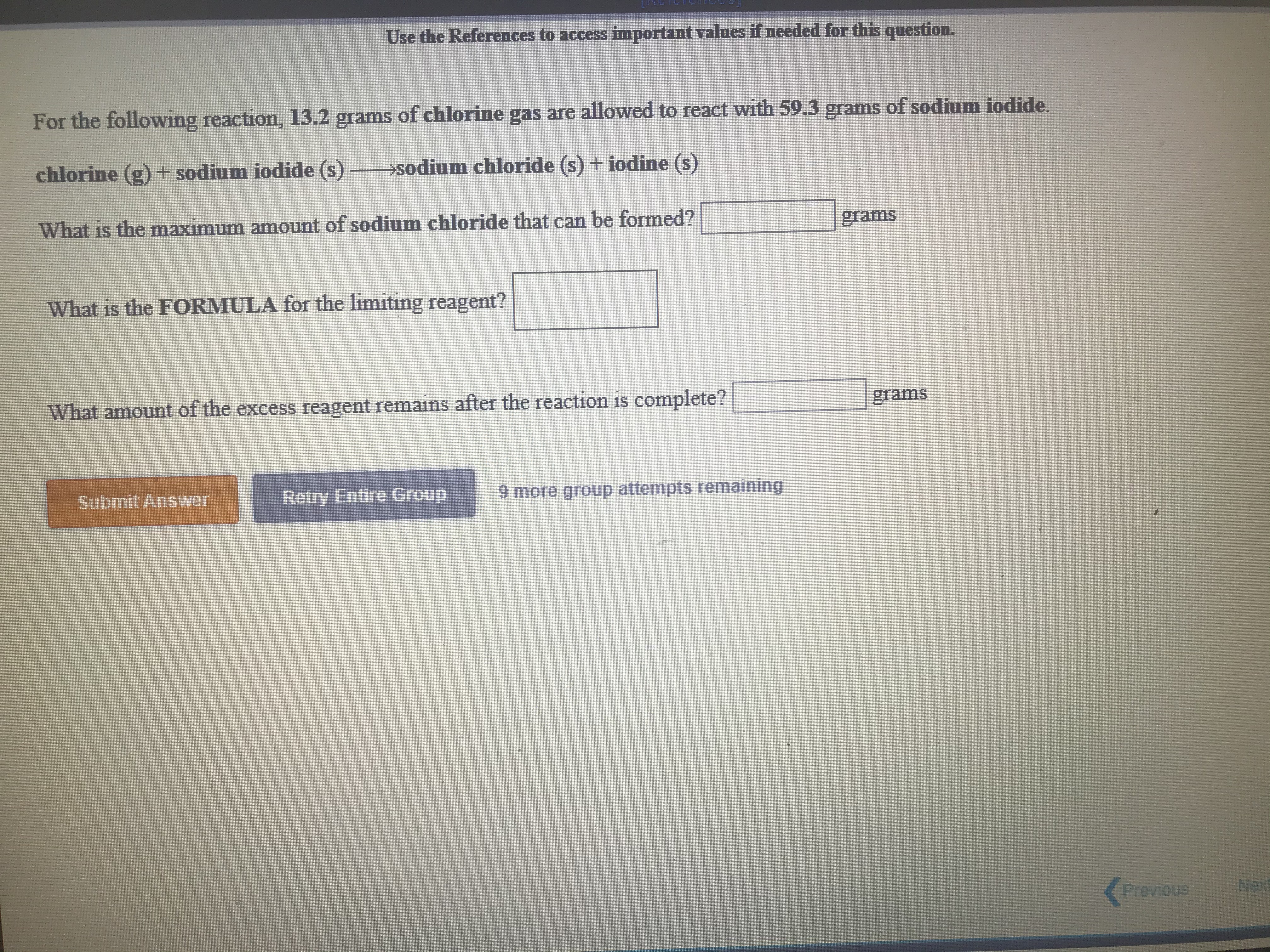
Transcribed Image Text:Use the References to access important values if needed for this question.
For the following reaction, 13.2 grams of chlorine gas are allowed to react with 59.3 grams of sodium iodide.
chlorine (g) + sodium iodide (s) sodium chloride (s) + iodine (s)
What is the maximum amount of sodium chloride that can be formed?
grams
What is the FORMULA for the limiting reagent?
1S
What amount of the excess reagent remains after the reaction is complete?
grams
Submit Answer
Retry Entire Group
9 more group attempts remaining
Previous
Nex
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- carbon monoxide (g) + oxygen (g) carbon dioxide (g) For the following reaction, 10.1 grams of carbon monoxide are allowed to react with 10.2 grams of oxygen gas. What is the maximum amount of carbon dioxide that can be formed? What is the FORMULA for the limiting reagent? What amount of the excess reagent remains after the reaction is complete?arrow_forwardFor the following reaction, 6.70 grams of benzene (C6H6) are mixed with excess oxygen gas. The reaction yields 18.0 grams of carbon dioxide. benzene (C6H6) (l) + oxygen (g) →→→carbon dioxide (g) + water (g) What is the theoretical yield of carbon dioxide ? What is the percent yield of carbon dioxide ? grams %arrow_forward4.0 g of iron(III) oxide (, molar mass = 160 g/mol) and 4.0 g of carbon monoxide (molar mass = 28 g/mol) are combined and allowed to react completely to form carbon dioxide and iron metal. The reaction occurs according to the following balanced chemical equation: Which is the limiting reactant?arrow_forward
- For the reaction shown, calculate how many grams of each product form when the following amounts of reactant completely react to form products. Assume that there is more than enough of the other reactant. 2Al(s)+Fe2O3(s)→Al2O3(s)+2Fe(l) -Calculate the mass of Al2O3 formed when 4.1 gAl completely react.Express your answer using two significant figures. -Calculate the mass of Fe formed when 4.1 gAl completely react. -Calculate the mass of Al2O3 formed when 4.1 gFe2O3 completely react.Express your answer using two significant figures. -Calculate the mass of Fe formed when 4.1 gFe2O3 completely react.Express your answer using two significant figures.arrow_forwardNitrogen dioxide is produced by combustion in an automobile engine. For the following reaction, 0.192 moles of nitrogen monoxide are mixed with 0.124 moles of oxygen gas. nitrogen monoxide(g)+oxygen(g)=nitrigen dioxide(g) What is the formula for the limiting reagent? What is the maximum amount of nitrogen dioxide that can be produced? molesarrow_forwardFor the following reaction, 19.3 grams of iron are allowed to react with 40.7 grams of chlorine gas .iron(s) + chlorine(g) iron(III) chloride(s)What is the maximum mass of iron(III) chloride that can be formed? grams What is the FORMULA for the limiting reagent? What mass of the excess reagent remains after the reaction is complete? gramsarrow_forward
- Use the References to access important values if needed for this question. For the following reaction, 26.8 grams of sulfur dioxide are allowed to react with 6.14 grams of water. sulfur dioxide(g) + water (1)→ sulfurous acid (H₂SO3)(g) What is the maximum mass of sulfurous acid (H₂SO3) that can be formed? g What is the FORMULA for the limiting reactant? Mass= What mass of the excess reagent remains after the reaction is complete? Mass= Submit Answer b.0 g Retry Entire Group Show Hint 8 more group attempts remaining Previous Next> Save and Exitarrow_forward3. Table salt (NaCl) is produced when sodium element reacts with chlorine gas. a) Write the balance chemical reaction. 2 Na +Cl2 2nacI b) Determine the mass of NaCl produced when 12.8 g of sodium reacts. c) Determine the mass of NaCl produced with 10.2 g of chlorine gas reacts. d) Which amount, 12.8 g of sodium or 10.2 g of chlorine gas, provides a better yield of NaCI?arrow_forwardFor the following reaction, 62.6 grams of iron(III) oxide are allowed to react with 25.4 grams of aluminum.iron(III) oxide (s) + aluminum (s) aluminum oxide (s) + iron (s)What is the maximum amount of aluminum oxide that can be formed? grams What is the FORMULA for the limiting reagent? What amount of the excess reagent remains after the reaction is complete? gramsarrow_forward
- For the following reaction, 10.6 grams of sodium are allowed to react with 5.70 grams of water .sodium ( s ) + water ( l ) sodium hydroxide ( aq ) + hydrogen ( g )What is the maximum amount of sodium hydroxide that can be formed? grams What is the FORMULA for the limiting reagent? What amount of the excess reagent remains after the reaction is complete? gramsarrow_forwardNitrogen dioxide is produced by combustion in an automobile engine. For the following reaction, 0.197 moles of nitrogen monoxide are mixed with 0.171 moles of oxygen gas. Nitrogen monoxide (G) + Oxygen (G) = Nitrogen dioxide (G) What is the formula for the limiting reactant? What is the maximum amount of nitrogen dioxide that can be produced? Amount = molarrow_forward= STOICHIOMETRY Limiting reactants A Aqueous hydrochloric acid (HCI) will react with solid sodium hydroxide (NaOH) to produce aqueous sodium chloride (NaCl) and liquid water (H₂O). Suppose 13. g of hydrochloric acid is mixed with 21.5 g of sodium hydroxide. Calculate the minimum mass of hydrochloric acid that could be left over by the chemical reaction. Be sure your answer has the correct number of significant digits. Explanation Check 99⁰ 0 X S VUDU Dany's 1/5 를 © 2023 McGraw Hill I All Rights Reserved. Terms of Use | Privacy Center Accarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY