
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Transcribed Image Text:A My Home x
O Alabama
V Activity
O Dashboard x
G is hbr ac
O (123) Ne
+
Assignment/takeCovalentActivity.
locatoreassionment-take
身 引ロ
(Review Topics
[References)
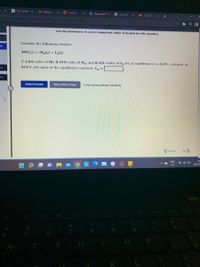
Use the References to access important values if needed for this question.
Consider the following reaction:
eq
2HI(g) H,(g) + I2(g)
If 1.64 moles of HI, 0.224 moles of H2, and 0.331 moles of I, are at equilibrium in a 12.9 L container at
914 K, the value of the equilibrium constant, Kp, is
be
eg
Submit Answer
Retry Entire Group
1 more group attempt remaining
(Previous
Next
ENG
21
令 口
US
5/5/20
7x
Home
End
8.
R
K
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Consider the following reaction:2HI(g) H2(g) + I2(g)If 2.04 moles of HI(g), 0.232 moles of H2, and 0.369 moles of I2 are at equilibrium in a 19.4 L container at 752 K, the value of the equilibrium constant, Kc, is .arrow_forwardConsider the following reaction: 2HF(g) ⟷H2(g) + F2(g) (K = 1.00 x10-2) Given 1.00 mole of HF(g), 0.500 mole of H2(g), and 0.750 mole of F2(g) are mixed in a 5.00-L flask, determine the reaction quotient, Q, and the net direction to achieve equilibrium.arrow_forwardThe equilibrium system shown below was analyzed and the concentrations of H2(g), I2(g) and HI(g), were found, in mol/L, to be 4.2, 3.8, 1.6 respectively. The equilibrium constant must be? H2(g) + I2(g) <=====> 2HI(g) + 65 kJarrow_forward
- Explain thoroughly and answerarrow_forwardUse the following information to answer the next question Factors that can affect equilibrium I and II III only I, II, and III I. O II only II. III. The factors from the list above that would increase the equilibrium concentration of NO(g) in the system; 4NH3(g) +502 (g) 4NO(g) + 6H₂O(g) AH = -905 kJ while all other variables are held constant are Decreasing the volume Increasing the temperature Adding NH3(g)arrow_forwardConsider the equilibrium system described by the chemical reaction below. If the partial pressures at equilibrium of NO, Cl2, and NOCI are 0.095 atm, 0.171 atm, and 0.28 atm, respectively, in a reaction vessel of 7.00 L at 500 K, what is the value of Kp for this reaction? 2 NO(g) + Cl2(g) = 2 NOCI(g)arrow_forward
- Consider the following reaction:2NOBr(g) 2NO(g) + Br2(g) If 0.224 moles of NOBr, 0.330 moles of NO, and 0.384 moles of Br2 are at equilibrium in a 14.0 L container at 518 K, the value of the equilibrium constant, Kp, is .arrow_forwardPlease don't provide handwritten solution ....arrow_forwardConsider the following reaction:COCl2(g) CO(g) + Cl2(g)If 2.98×10-2 moles of COCl2, 0.206 moles of CO, and 0.304 moles of Cl2 are at equilibrium in a 17.8 L container at 672 K, the value of the equilibrium constant, Kp, is .arrow_forward
- Consider the following reaction:COCl2(g) CO(g) + Cl2(g)If 8.62×10-3 moles of COCl2, 0.353 moles of CO, and 0.282 moles of Cl2 are at equilibrium in a 12.5 L container at 757 K, the value of the equilibrium constant, Kp, is .arrow_forwardType the word True or False in the provided space for the following. Consider the following system at equilibrium where Kc = 9.52x10-2 and AH° = 18.8 kJ/mol at 350 K. CH4 (g) + CCl4 (g) → 2 CH₂Cl₂ (g) The production of CH₂Cl2 (g) is favored by: decreasing the temperature. increasing the pressure. increasing the volume. removing CH₂Cl2. removing CCl4. I. II. III.____ IV. V.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY