
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
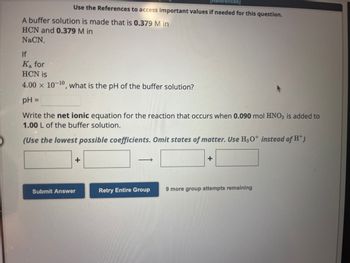
Transcribed Image Text:Use the References to access important values if needed for this question.
A buffer solution is made that is 0.379 M in
HCN and 0.379 M in
NaCN.
If
Ka for
HCN is
4.00 x 10-¹0, what is the pH of the buffer solution?
pH =
Write the net ionic equation for the reaction that occurs when 0.090 mol HNO3 is added to
1.00 L of the buffer solution.
(Use the lowest possible coefficients. Omit states of matter. Use H3O+ instead of H+)
+
Submit Answer
->>
Retry Entire Group
+
9 more group attempts remaining
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A 76.0 mL sample of 0.0100 M HIO4 is titrated with 0.0200 M RbOH solution. Calculate the pH after the following volumes of base have been added. (a) 14.1 mL pH = (d) 39.9 mL pH = (b) 37.2 mL pH = (e) 69.5 mL pH = (c) 38.0 mL pH =arrow_forwardYou have 1.5 liter of solution that is composed of 8.88 grams of NH3 and 11.33 grams of ammonium chloride mixed well. Kb for ammonia =1.8 x 10^-5 (A) is this a buffer solution? Why or why not? (B)if it is a buffer solution, what is the pH of this buffer solution? (C)how many mL of 1.50 M HCl can be added to this solution before the buffer is exhausted (d) how many mL of 1.5 M NaOH can be added to this solution before the buffer is exhausted?arrow_forwardDetermine the pH during the titration of 59.7 mL of 0.392 M hypochlorous acid (K₂ = 3.5×10-8) by 0.392 M KOH at the following points. (Assume the titration is done at 25 °C.) (a) Before the addition of any KOH (b) After the addition of 13.0 mL of KOH (c) At the half-equivalence point (the titration midpoint) (d) At the equivalence point (e) After the addition of 89.6 mL of KOH Submit Show Approach Show Tutor Stepsarrow_forward
- A buffer solution is made that is 0.415 M in CH3COOH and 0.415 M in CH3COO- . (1) If Ka for CH3COOH is 1.80×10-5 , what is the pH of the buffer solution? (2) Write the net ionic equation for the reaction that occurs when 0.103 mol HI is added to 1.00 L of the buffer solution. Use H3O+ instead of H+ .arrow_forwardA 81.0 mL sample of 0.0500 M HBrO4 is titrated with 0.100 M NaOH solution. Calculate the pH after the following volumes of base have been added. (a) 14.2 mLpH = (b) 39.3 mLpH = (c) 40.5 mLpH = (d) 42.5 mLpH = (e) 79.0 mLpH =arrow_forwardA buffer solution is made that is 0.394 M in H2S and 0.394 M in NaHS. If Ka1 for H2S is 1.00 x 10^-7, what is the pH of the buffer solution? pH = Write the net ionic equation for the reaction that occurs when 0.098 mol HNO3 is added to 1.00 L of the buffer solution. (Use the lowest possible coefficients. Omit states of matter. Use instead of )arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY