
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
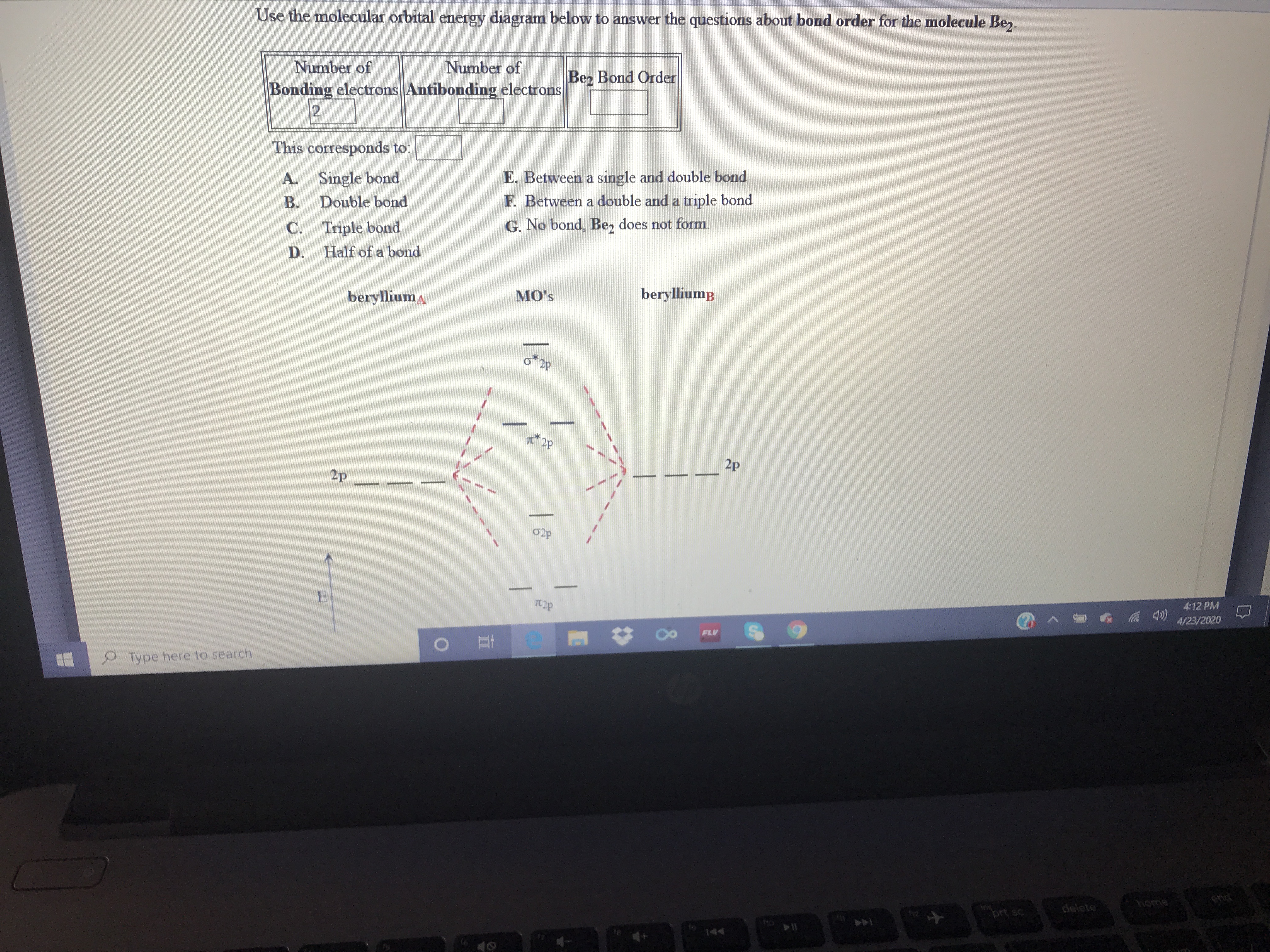
Transcribed Image Text:Use the molecular orbital energy diagram below to answer the questions about bond order for the molecule Be,.
Number of
Bonding electrons Antibonding electrons
12
Number of
Be, Bond Order
This corresponds to:
A. Single bond
Double bond
E. Between a single and double bond
F. Between a double and a triple bond
B.
C. Triple bond
G. No bond, Be, does not form.
D.
Half of a bond
berylliuma
MO's
berylliump
2p
2p
02p
E.
4:12 PM
4/23/2020
FLV
Type here to search
home
end
prt sc
144
Transcribed Image Text:hline teachin X
https://cvg.cengagenow.com/ilm/takeAssignment/takeCXPCompliantActivity.do?locator=assignment-take&takeAssignmentSessionLocator=assignment-take
To see favorites here, select then , and drag to the Favorites Bar folder. Or import from another browser. Import favorites
I2p
2p
2p
Op
o*23
- 2s
o*1s
1s
Try Another Version
Submit Answer
FLV
Type here to search
| 5
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 7 images

Knowledge Booster
Similar questions
- What’s the answer I only want the answerarrow_forwardme File Edit View History Bookmarks Profiles Tab Window Help N Watch Gilmore Girls | Netflix a ALEKS A ALEKS - Reyna Garcia - Learn X O Psychology Research Sign-Up x T WPAL 101233 Spring 2022 www-awu.aleks.com/alekscgi/x/lsl.exe/1o_u-IgNslkr7j8P3jH-IvdWKW_BBZZ16tTytly4Fcfu6zOtOf8oMM9sqG0YAPcLeyNqu8U3rAMkvKt93Vpt7xcKoyt... O * O Spotify Web Playe.. M common Ethical D. O CHEMICAL REACTIONS R 0/5 Solving for a reactant using a chemical equation A major component of gasoline is octane (C,H18). When octane is burned in air, it chemically reacts with oxygen gas (O,) to produce carbon dioxide (CO,) (H,). and water What mass of oxygen gas is consumed by the reaction of 4.84 g of octane? Be sure your answer has the correct number of significant digits. Ac Check Explanation O 2022 McGraw Hill LLC. All Rights Reserved. Terms of Use Privacy Center IIarrow_forwardplease help me answer this one, thanks.arrow_forward
- Success Confirmation of Questio X → C C D RC Email VConnect ✪ ← + New ♥ ✩ B ☆ IMG_1333.heic My Drive Computers drive.google.com/drive/u/0/recent?lfhs=2 VConnect Search List ➡ Screening Appoint... ✪ Opportunity Queue Shared with me Recent Starred Trash Storage 27.98 GB of 100 GB used New Tab Buy storage a Search in Drive - Recent - Google Drive A. 13 Before you answer this question, please read the following important principle: H₂C=CHCH₂CH₂CH₂ D. CH₂ If a carbocation is formed in the slow step of a reaction, the reactivity of the reactant will be greater if that carbocation is more stable. The question: Which of the following alkenes reacts fastest with HCI? CH₂CH₂ H H RC RRL Google My Drive > IMG_1333.heic Open with O B. H₂C- CH₂ Google Docs in Elms College -C-CH=CH₂ H + N C. H₂C H an H 0 32 CH₂CH₂ O Ⓒ US : 0 |+ IMG_1344.heic Dec 16 19 ex 1:20 → → ↓ : **** ⠀ +arrow_forward- Discord | #-courtyar → C Smail Official Miami Dade College x www-awu.aleks.com/alekscgi/x/Isl.exe/1o_u-IgNslkr7j8P3jH-UibwdleAUz2IYCcR4NM7yr_IpMriNwxObE8BNBktcRGBtTHK1XmgWvpNI8wuDkZtcqnDqEtC1XWgHvKTc... ☆ YouTube Translate O KINETICS AND EQUILIBRIUM Calculating the change in concentration after a whole number o... y! mdc.edu - Yahoo Search Resu X Hg mL Explanation 83°F Mostly sunny The rate at which a certain drug is eliminated by the body follows first-order kinetics, with a half life of 28 minutes. Suppose in a particular patient the concentration of this drug in the bloodstream immediately after injection is 0.47 ug/mL. What will the concentration be 140 minutes later? Round your answer to 2 significant digits. Check x10 X H 5 MHCampus/Connect(ALEKS) X A ALEKS- Mia Reboredo - Lear X Q Search LO 4/5 + A k © 2023 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Acc % 040 coarrow_forwardLab Exam 1- Requires Respon X 021 Summary - CHM151-N803: Ger X A ALEKS - Delana Bailey - Learn X C Sign In or Sign Up | Chegg.com X www-awu.aleks.com/alekscgi/x/lsl.exe/1o_u-IgNslkr7j8P3jH-IJgg7xIASweFMYphv4tCLy6BMpSgONHO-bYrHeBZ4S54EqYg5AJLeyt82s1i-_fz32exQQdKy96vMC... ☆ → C tab caps lock shift O CHEMICAL REACTIONS Solving for a reactant using a chemical equation Ammonium phosphate ((NH4),PO4) What mass of ammonium phosphate is produced by the reaction of 7.4 g of ammonia? Round your answer to 2 significant digits. esc Explanation ! 1 Q e A 1 control option @ 2 N Check W S #3 is an important ingredient in many fertilizers. It can be made by reacting phosphoric acid (H₂PO4) with ammonia (NH₂). X H command E D X $ 4 C R FL do 5 % V T MacBook Pro 6 Y & 7 G H B Ⓒ2022 McGraw Hill LLC. All Rights Reserved. U 8 J | ΤΝΤ Μ N Nitrogen N2 gas and hydrogen X + ( 9 K 0 ) 0 Terms of Use | Privacy Center | Accessibility L 0/5 P , | 1 command option WE { [ + 11 Delana 2 | 000 Ar KI } ? D Update…arrow_forward
- MyCSU-Columbus State Univers X M Inbox (1) - bailes_amber@colum X D2L Quiz 1- Intro Resrch Meth in CJ K ← → CO 03 Email MGmail X A ALEKS-Amber Bailes - Learn X + www-awu.aleks.com/alekscgi/x/Isl.exe/1o_u-IgNslkr7j8P3jH-IJgXZp57itWHhRgilODc5MqvhZbKYx2-U-03700TYd4ABbH6KAJLiK5JfbHdk1X12WQ... LE YouTube Maps MyCSU-Columbus... B Homepage - Georg... Microsoft Office Ho... BLesson 2 Discussion... III MyMidland Mortga... CHEMICAL REACTIONS Solving for a reactant using a chemical equation 3/5 Green plants use light from the Sun to drive photosynthesis. Photosynthesis is a chemical reaction in which water (H₂O) and carbon dioxide (CO₂) chemically react to form the simple sugar glucose (C6H₁2O6) and oxygen gas (0₂). 12 What mass of carbon dioxide is consumed by the reaction of 3.6 g of water? Be sure your answer has the correct number of significant digits. g x10 Ś ? Explanation Ⓒ2022 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Ce 78°F O Et C ||| Check Type here to search X a no…arrow_forwardHelp 100% 47 T. "ublic Health Ch HSC 258 - Major Projec x MindTap - Cengage Lea X C The Illustration To T =55750828934189288909969212&elSBN=9781305657571&id=D1061392007&nbld=21... * Q Search t Referonces Use the References to access important values if needed for this question. For the following reaction, 50.4 grams of sulfur dioxide are allowed to react with 17.9 grams of water. sulfur dioxide (g) + water (I) sulfurous acid (H2SO3) (g) grams What is the maximum amount of sulfurous acid (H,SO3) that can be formed? What is the FORMULA for the limiting reagent? grams What amount of the excess reagent remains after the reaction is complete? Submit Answerarrow_forwardA S19 3.4 "Х S19 3.4 . Cengage | M Inbox (112)-| MindTap × I+ ttps:// ng.cengage.com/static/nb/ui/evo/index.html?e ISBN #9781 305 65757 1 &id-4309342 66&snapshot id-1 065250& INDTAP Q search this course stions 2NO(g) +02(g) 2NO2(g) It is observed that, when the concentration of O2 is reduced to 1/4 of its initial value, the rate of the reaction is also reduced to 1/4 of its initial value, When the concentration of NO is multiplied by 6.13, the rate of the reaction increases by a factor of 37.6. (a) Write the rate expression for this reaction, and give the units of the rate constant k, assuming concentration is expressed as mol L and time is in seconds rate = k units- (b) If INO] were multiplied by 4.91 and [O2] by 2.56, what change in the rate would be observed? The rate would increase by a factor of Next Autosaved at 9:31 AM Back 9:31 Alv 2/28/201arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY