
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
complete the rows 1-4 column of the table
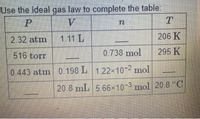
Transcribed Image Text:Use the ideal gas law to complete the table:
P
V.
2.32 atm
1.11 L
206 K
516 torr
0.738 mol
295 K
0.443 atm 0.198 L 122×10-2 mol
20.8 mL 5.66x10-3 mol 20.8 °C
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Determine the answer to the following problem. Make sure your final answer has the proper number of significant figures. 15.67200−33.009000arrow_forward%23 Laboratory Experiments 15 QUESTIONS 1. Identify each of the following as measurements of length, area, volume, mass, density, time, or temperature: ³. (a) ns, (b) 10.0 kg/m³, (c) 1.2 pm, (d) 750 km², (e) 83 K, and (f) 4.0 mmarrow_forwardPlot the data appropriately on a properly formatted graph. Formatting should include descriptive title, axes labels with units, major grid lines marked, proper scaling, and a best fit line (use a ruler).arrow_forward
- Answer the questions below by circling the number of the correct response The symbol for potassium is (1) P, (2) K, (3) Sn, (4)Po. A substance that cannot be broken down into simpler substances during a chemical reaction is a[n] (1) element, (2) compound, (3) mixture, (4) none of these. Which of the following is NOT an element? (1) oxygen (2) nitrogen (3) lead (4) sodium chloride Which of the following is the correct symbol for copper? (1) Co (2) Cu (3) CU (4) cu The smallest particle of an element is a[n] (1) atom, (2) molecule, (3) compound, (4) ion. Water can be broken down into hydrogen and oxygen. This is evidence that water is NOT a[n] (1) compound, (2) molecule, (3) element, (4) mixture. The symbol for silver is (1) Si, (2) S, (3) Au, (4) Ag Hg is the symbol for (1) hydrogen, (2) mercury, (3) tin, (4) sodium. The symbol for cadmium is (1) Cd, (2) Ca, (3) C, (4) Cm. Mg is the symbol for (1) manganese, (2) mercury, (3) silver, (4) magnesium. An example of a substance…arrow_forwardUse the graph below to answer the following questions. Assume one atom produces one count per minute. Do not include units in your answers. Counts per minute 80 70 60 50 40 30 20 10 0 0 1 2 3 4 5 Time (Days) 6 7 8 9 10arrow_forward26 1 3. 12M.+ On → le+ 27 12M9 26 11NA 27 iiNa 11N. 27 13ALarrow_forward
- An electric current of 22.90 A flows for 20.0 minutes. Calculate the amount of electric charge transported. Be sure your answer has the correct unit symbol and the correct number of significant digits.arrow_forwardNp=Ne Express as an integer, the element is manganesearrow_forward13.3 x 10-5 kiloliters to microlitersarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY