
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
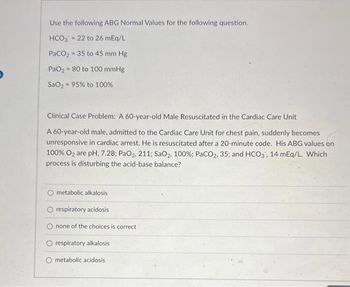
Transcribed Image Text:Use the following ABG Normal Values for the following question.
HCO3 = 22 to 26 mEq/L
PaCO₂ - 35 to 45 mm Hg
PaO₂80 to 100 mmHg
SaO, = 95% to 100%
Clinical Case Problem: A 60-year-old Male Resuscitated in the Cardiac Care Unit
A 60-year-old male, admitted to the Cardiac Care Unit for chest pain, suddenly becomes
unresponsive in cardiac arrest. He is resuscitated after a 20-minute code. His ABG values on
100% O₂ are pH, 7.28; PaO2, 211; SaO2, 100%; PaCO2, 35; and HCO3, 14 mEq/L. Which
process is disturbing the acid-base balance?
O metabolic alkalosis
O respiratory acidosis
none of the choices is correct.
O respiratory alkalosis
metabolic acidosis
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- An action will elevate the concentrations of three chemicals in the drinking water supply: 1,1,1- trichloroethane to 2 mg/L, tetrachloroethylene to 0.04 mg/L and 1,1-dichloroethylene to 0.1 mg/L. Determine the lifetime risk of developing an undesirable health effect due to this exposure for a 50 kg adult who drinks 1 L/day of water containing these elevated pollution levels. Is there cause for concern? Why?arrow_forwardConsider the neutralization reaction 2HNO3(aq)+Ba(OH)2(aq)⟶2H2O(l)+Ba(NO3)2(aq)2HNO3(aq)+Ba(OH)2(aq)⟶2H2O(l)+Ba(NO3)2(aq) A 0.110 L0.110 L sample of an unknown HNO3HNO3 solution required 54.5 mL54.5 mL of 0.250 M Ba(OH)20.250 M Ba(OH)2 for complete neutralization. What is the concentration of the HNO3HNO3 solution?arrow_forwardQ11 chapter 15 preparing solutions Solve Asap pleasearrow_forward
- JJ is making miso soup. 1 bowl of soup has a volume of 375 mL. The soup has 1.00 g of table salt in it (NaCl). What is the salt concentration of the soup in g/L?arrow_forwardIf a pOH meter is placed in a 0.00300 mol/L solution of nitric acid, the POH reading would be: O 3.00 O 11.5 O 0.500 O 0.300 O 2.50arrow_forwardThe recommended dose of Remdesivir is 200 mg (0.200 grams) by injection on day 1. Assuming the blood volume of the average adult is 5 liters, and the molecular weight of Remdesivir is 602.56 g/mole, what is the molarity of Remdesivir in moles/liter? The recommended dose of Remdesivir is 200 mg (0.200 grams) by injection on day 1. Assuming the blood volume of the average adult is 5 liters, and the molecular weight of Remdesivir is 602.56 g/mole, what is the molarity of Remdesivir in moles/liter? 9.2 x 10-6 M 1.0 x 10-3 M 6.64x 10-5 M 4.0 x 10-5 Marrow_forward
- 43.27 mL of 0.5033 M NaOH are required to neutralize 29.16 mL of a H2SO4 solution.What is the molarity of the H2SO4 solution? H2SO4 + 2 NaOH ® Na2SO4 + 2 H2Oarrow_forwardYou are asked to prepare a 1.000 L solution of 4.5 M you commit a user error while preparing this solution. Assumed volume Volumetric error Preparation details Added water 2.0 cm above the line, which corresponds to 8.2 mL (0.0082 L) additional solution volume C6H12O6 (glucose; molar mass = 180.16 g/mol) in a lab by dissolving 811.0 g of glucose in water. Consider the following two scenarios in whic Prepared in a beaker Prepared in a volumetric flask 1.000 L You add the glucose to a volumetric flask and then add water until it dissolves. The water bottle you are using has a worn tip, and you inadvertently add too much water such that the meniscus is above the line. The diameter of the neck of the volumetric flask is 2.29 cm. 811.0 g glucose Concentration: You decide to evaluate and compare the errors you made while preparing the solutions using the different methods. Calculate the actual concentrations of the intended 4.5 M glucose solutions prepared by each method based on their…arrow_forwardTo accurately prepare a standard solution, the correct glassware must be used to perform the dilution. What glassware is required to prepare a standard solution from a solid? Choose the most correct answer. beaker, conical flask, Pasteur pipette (dropper) beaker, volumetric flask, conical flask beaker, volumetric flask, Pasteur pipette (dropper) Pipette, volumetric flask, Pasteur pipette (dropper) beaker, measuring cyclinder, Pasteur pipette (dropper)arrow_forward
- Consider the neutralization reaction 2HNO3(aq)+Ba(OH)2(aq)⟶2H2O(l)+Ba(NO3)2(aq)2HNO3(aq)+Ba(OH)2(aq)⟶2H2O(l)+Ba(NO3)2(aq) A 0.125 L0.125 L sample of an unknown HNO3HNO3 solution required 54.3 mL54.3 mL of 0.200 M Ba(OH)20.200 M Ba(OH)2 for complete neutralization. What is the concentration of the HNO3HNO3 solution?arrow_forwardWhat mass of CaCO3 would be needed to neutralize 5.45 mL of 7.979 M HCl? (Molar mass of CaCO3 is 100.09 g/mol) Report your answer with two decimals. Enter numbers only; do not enter units.arrow_forwardA doctor has ordered for a week 0.35 grams of Carvedilol for a patient with high blood pressure. If your stock on hand consists of 25 mg tablets, how many tablets will you need for 1 day's treatment for the patient? QUESTION 13 O a. 2 O b. 14 O C. 7 O d. 20 O e. 1 QUESTION 14arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY