
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
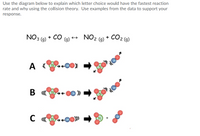
Transcribed Image Text:Use the diagram below to explain which letter choice would have the fastest reaction
rate and why using the collision theory. Use examples from the data to support your
response.
NO3 (9) + CO (9)
2 (9) + CO2 (9)
A
A {+00E
В «
B
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 4 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- From the data given in the table below, determine the rate law for the following chemical equation. NH4+(aq) + NO2-(aq) → N2(g) + 2 H2O(l) Experiment [NH4+]o [NO2-]o Initial Rate (consumption of NH4+) 1 0.24 0.10 7.2 x 10-6 M/s 2 0.12 0.10 3.6 x 10-6 M/s 3 0.12 0.15 5.4 x 10-6 M/s Select one: a. Rate = k[NH4+][NO2-]2 b. Rate = k[NH4+]2[NO2-] c. Rate = k[NH4+]2 d. Rate =k[NH4+]2[NO2-]2 e. Rate = k[NO2-] f. Rate = k[NO2-]2 g. Rate = k[NH4+] h. Rate = k[NH4+][NO2-]arrow_forwardTIAarrow_forwardConsider the reaction: H2 (g) +I2 (g) → 2 HI (g) A chemist performed an experiment and monitored the concentration of I2 during the course of the reaction. The red line in the graph below represents the results obtained. Which line in the plot would best represent how the concentration of HI changes during the course of the reaction? Time (s) Select an answer and submit. For keyboard navigation, use the up/down arrow keys to select an answer. a a b b d d е е Concentration (M)arrow_forward
- need help with this chemistry correct sig-figarrow_forwardGiven the following data, determine the rate constant (in M-2s-1) of the reaction 2NO(g) + Cl2(g) –→ 2NOCI(g) [Cl2] (M) Rate (M/s) 3.4 x 104 8.5 x 105 3.4 x 104 Experiment [NO] (M) 1 0.0300 0.0100 0.0150 0.0100 3 0.0150 0.0400 Note: Answers must be numeric, not alphanumeric-42, not forty-two.arrow_forward(a) Select all of the correct statements about reaction rates from the choices below. The lower the rate of a reaction the longer it takes to reach completion. A balanced chemical reaction is necessary to relate the rate of reaction to the concentration of a reactant. As a reaction progresses its rate goes down. The rate of a slow step has more effect on the overall reaction rate than the rate of a fast step. Reaction rates are determined by reactant concentrations, temperatures, and reactant stabilities. Reaction rates increase with increasing temperature. Reactions involving very unstable combinations of chemicals have large rate constants.arrow_forward
- The data below were collected for the following reaction: CH3CI(g) + 3 Cl2(g) CCI4(g) + 3 HCI(g) [CH3CI] / mol L-1 Initial Rate / mol L-1 s-1 Trial [Cl2] / mol L-1 1 0.050 0.050 0.0142 2 0.100 0.050 0.0290 3 0.200 0.200 0.115 What is the order of the reaction with respect to Cl2?arrow_forwardUsing the data in the table, calculate the rate constant of Trial [A] (M) [B] (M) Rate (M/s) this reaction. 1 0.260 0.280 0.0119 A + B → C+ D 2 0.260 0.784 0.0933 3 0.312 0.280 0.0143 Units k = M-²s-1 M-'s-! M-s-!arrow_forwardCalculate the average rate of the reaction between 10 and 20 seconds.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY