
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
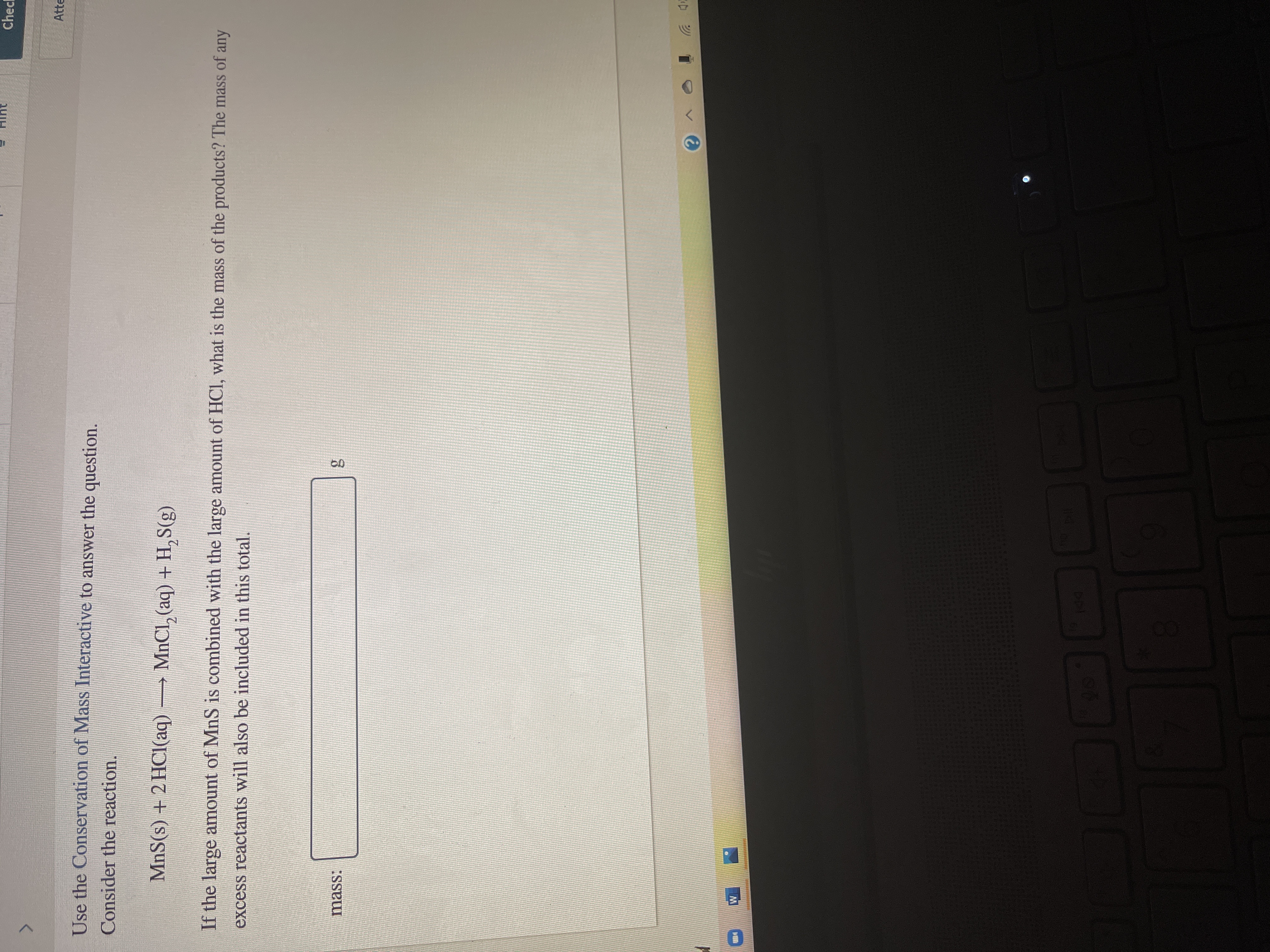
Use the conservation of mass interactive to answer the questions. Consider the reaction.
MnS(s) + 2 HCI(aq) __ MnCI2(aq) +H2S(g)
if the large amount of MnS is combined with large amount HCI, what is the mass of the products? The mass of any excess reaction will also be included in this total
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 1-Classify each chemical compound: compound type of compound Ca(CN)2 ___ ionic ___molecular Cr3P2 ___ ionic ___molecular PCl5 ___ ionic ___molecular 2-Classify each chemical reaction: Reaction Type K2SO4(aq) + Ba(NO3)2(aq) → 2KNO3 (aq) + BaSO4 (s) choose one ____combination ____decomposition ___single substitution ___double substitution ___none of the above 2KNO3 (s) → 2KNO2 (s) + O2 (g) choose one ____combination ____decomposition ___single substitution ___double substitution ___none of the above Cl2 (g) + CuBr2 (aq) → CuCl2 (aq) + Br2 (l) choose one ____combination ____decomposition ___single substitution ___double substitution ___none of the above 2Na (s) + F2 (g) → 2NaF (s) choose one ____combination ____decomposition ___single substitution ___double substitution ___none of the above 3-Multiply or divide the following measurements. Be sure each answer you enter contains the correct number of…arrow_forwardComplete and balance the following molecular reactions. Write the ionic and net ionic reactions. ___ K3PO4 (aq) + ___ CuCl2 (aq) =arrow_forwardComplete (where necessary) and balance the following equation. Write the stoichiometric equation, then write the equation in ionic and net ionic form. __ CuO (s) + __ H2SO4 (aq) → __ CuSO4 (aq) + __________arrow_forward
- Complete the table below by deciding whether a precipitate forms when aqueous solutions A and B are mixed. If a precipitate will form, enter its empirical formula in the last column. Does a precipitate form when A and B empirical formula of precipitate solution A solution B are mixed? sodium hydroxide copper(II) bromide O yes O no sodium chloride ammonium bromide O yes O no ammonium sulfide zinc bromide O yes O noarrow_forwardComplete the table below by deciding whether a precipitate forms when aqueous solutions A and B are mixed. If a precipitate will form, enter its empirical formula in the last column. Does a precipitate form when A and B empirical formula of solution A solution B precipitate are mixed? zinc nitrate sodium hydroxide yes no iron(II) bromide zinc acetate yes no iron(II) chloride ammonium sulfide yes no Acces Check O 2021 McGraw Hill LLC. All Rights Reserved. Terms of Use Privacy Center Explanation MacBook Proarrow_forwardComplete the table below by deciding whether a precipitate forms when aqueous solutions A and B are mixed. If a precipitate will form, enter its empirical formula in the last column. Does a empirical formula of precipitate form when A and B are mixed? solution A solution B precipitate manganese(II) bromide zinc nitrate O yes O no sodium sulfide O yes copper(II) sulfate no sodium hydroxide zinc nitrate O yes O noarrow_forward
- If 20.6 g of Nal (MM = 149.89 g/ mol) are added to a 500.0 mL volumetric flask, and water is added to fill the flask, what is the concentration of Nal in the resulting solution?arrow_forward14. A student wants to determine the concentration of a AgNO3 solution. 200.0 mL of the AgNO3 solution is reacted with an aqueous NazSO4 solution until no more precipitate forms. A total of 5.4 grams of precipitate formed. What is the molarity of the AgNO3 solution? 2AGNO3(aq) + NazSO4(aq) 2NANO3(aq) + Ag:SO:(s) A) 0.085 M B) 0.17 M 1 C) 0.35 M D) 0.70 M E) 1.3 Marrow_forwardPlease don't provide handwritten solution .....arrow_forward
- What mass of iron(III) hydroxide precipitate can be produced by reacting 97.0 mL of 0.250 M iron(III) nitrate with 179 mL of 0.260 M sodium hydroxide? Mass= __garrow_forward1.0 chem qarrow_forwardComplete the table below by deciding whether a precipitate forms when aqueous solutions A and B are mixed. If a precipitate will form, enter its empirical formula in the last column. Does a empirical formula of precipitate form when A andB are mixed? solution A solution B precipitate potassium acetate barium nitrate O yes O no iron(II) nitrate potassium sulfide O yes O no zinc nitrate sodium hydroxide O yes O noarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY