
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Use the centimeter ruler and the gray object below to indicate how many certain and uncertain digits the length measurement should have.
Group of answer choices
Number of certain digits = 3; Number of uncertain digits = 0
Number of certain digits = 3; Number of uncertain digits = 1
Number of certain digits = 2; Number of uncertain digits = 1
Number of certain digits = 2; Number of uncertain digits = 0
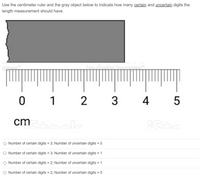
Transcribed Image Text:Use the centimeter ruler and the gray object below to indicate how many certain and uncertain digits the
length measurement should have.
ㅇ
1
3
4 5
cm k
Number of certain digits = 3; Number of uncertain digits = 0
O Number of certain digits = 3; Number of uncertain digits = 1
O Number of certain digits = 2; Number of uncertain digits = 1
O Number of certain digits = 2; Number of uncertain digits = 0
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Add or subtract the following measurements. Be sure each answer you enter contains the correct number of significant digits. 5.700 mL 4.60 mL - = 16.6 mL +1.57 mL = 18.80 mL + 15.577 mL = mL mL mL x10 X Sarrow_forwardUsing rules for significant figures in calculations, divide 0.39 by the number 5.12. Choose the answer with the correct number of sig figs. 0.076 0.08 0.0762 0.076171875 Question 2 Choose the best conversion of 887.913 to scientific notation with three significant figures. 0.888 x 103 1080- acerarrow_forwardX₂ X™ Ω- Perform the following operations giving the proper number of significant figures in the answer: 23.4 x 14=arrow_forward
- Change the following measurement to the appropriate SI unit. The final unit required is shown to the right of the answer box. Be sure to use correct significant figures. An automobile engine with a displacement of 4.16×102 in.3 = cm3arrow_forwardPerform the following calculations: a) 14.86 ml + 15.0 ml + 14.980 ml = b) (42.927 g/ml)(9.00 ml) = Round off the answers to the proper significant figures.arrow_forwardAccurate? Precise? Density= Measurement, Significant Figures, Rounding, Precision and Accuracy 1. How many significant figures in each of the following measurements? 3.121 m 4 2 1.4 X 104g. 2 0.051 L 120 ft 2. Round the following numbers to 3 significant figures. 2.35500 23 9.385 9.59 2 Group A 12.348 g 12.441 g 12.367 g 12.392 g 3. Solve the following and express the answer in the correct number of significant figures. a. The average mass of 3 objects whose individual masses are 10.3 g, 9.334 g, 9.25 g. 10.39 + 9.334 + 9.25 +3= 28.8849, or 28.9 b. The volume of a metal block whose dimensions are 2.44 cm by 4.2 cm by 3.571 cm. 2.44 4.2.3.54= 36.60 Group B 12.412 g 12.420 g 12.418 g 12.415 g 0.0150 L 3 3 200. K c. Carry out the following operations and express your answer in the correct number of significant figures. (12.8+ 12.71)/0.560 = 45.55 155./(43.25-7.1) = 4,288 4. Four groups of students where charged with measuring the mass of 10.0 mL of a solution of sugar water and from there…arrow_forward
- Add or subtract the following measurements. Be sure each answer you enter contains the correct number of significant digits. 9.677g-1.30g 3.877g-1.40g 11.500g+0.9garrow_forwardChange the following measurement to the appropriate SI unit. The final unit required is shown to the right of the answer box. Be sure to use correct significant figures. An automobile engine with a displacement of 4.36×102 in.3 = cm3 Change the following measurement to the appropriate SI unit. The final unit required is shown to the right of the answer box. Be sure to use correct significant figures. 221 lb man = kg Change the following measurement to the appropriate SI unit. The final unit required is shown to the right of the answer box. Be sure to use correct significant figures. 18643 ft elevation above sea level = marrow_forwardAdd or subtract the following measurements. Be sure each answer you enter contains the correct number of significant digits. 19.770 g 0.9 g 6.50 g +1.500 g 6.50 g - 0.6 g = = 8 x10 X Undoarrow_forward
- 1. What is the length of the rod shown below? 3 4 7 10 2. If Student A measures the volume of a liquid and reports its volume as 23.50 mL and Student B measures a different amount of the same liquid and reports its volume as 15.0 mL, is it likely that the students used the same measuring device? Yes or no? Explain your answer. 3. If you were asked to measure 25.0 mL of a liquid and to transfer it to another container, which of the following glassware could you use? Choose all that apply. Give reason(s). a) A 50-mL buret with smallest divisions of 0.1 mL b) A 50 mL beaker with smallest divisions of 10 mL c) A 50-mL volumetric flask d) A 50-mL graduated cylinder with smallest divisions of 1 mL e) A 25-mL pipet 4. A group of five students attempted to estimate 100 g of a substance by balancing the amount of the substance with a 100-g standard mass and obtained the following masses after each estimated amount was weighed: 95.8634 g, 80.8125 g, 106.5078 98.2865 and 86.4453 Create a data…arrow_forwardIn the summer, the outdoor temperature in the Northeast might be 84°F. What is this temperature on the Celsius and Kelvin scales? Temperature = Temperature °C Karrow_forwardPerform the following calculation and round answer to the correct number of significant figures? (2.17cmx3.22cm)+1.8cm. 9.00cm 8.8cm 9cm 8.79cmarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY