Question
thumb_up100%
Use the binding energy per nucleon curve in the figure above to determine how much energy must be supplied to separate the nucleons in a (140/58)Ce nucleus.
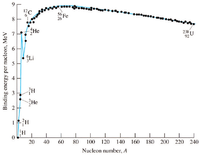
Transcribed Image Text:9.
56 Fe
26
8
He
238 U
92
ŞLi
He
60
80
100
120 140 160 180
200 220 240
20
40
Nucleon number, A
3.
2.
Binding energy per nucleon, MeV
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- The atomic mass of 14C is 14003242 u. Show that β- decay of 14C is energetically possible, and calculate the energy released in the decay.arrow_forwardCalculate the average binding energy per nucleon of the phosphours-31 nucleus, P. 15arrow_forwardWhat is the approximate radius of a 112/48 Cd nucleus?arrow_forward
- Calculate the binding energy per nucleon for 232Th, ¹2C, 78Se, and 5⁹ Co. (For the atomic masses, see this table. Enter your answers to at least two decimal places.) (a) 232Th (b) 12C (c) (d) 78 Se 59 CO MeV/nucleon MeV/nucleon MeV/nucleon MeV/nucleonarrow_forwardA 23994Pu isotope is isolated from a lab sample. (a) What is the nuclear radius (in fm)? (b)If a hypothetical isotope with triple the radius (found in part (a)) were isolated from the sample, what would its mass number be?arrow_forwardRadon 22Rn produces a daughter nucleus that is radioactive. The daughter, in turn, produces its own radioactive daughter, and so on. This process continues until lead 208Pb is reached. What are the total number No of a particles and the total number No- of ß* particles that are generated in this series of radioactive decays? Na = Number i NB- - = Number i Units Unitsarrow_forward
- Calculate the binding energy for the following nuclides; expressd the binding energy in: (i) J/nucleon, and (ii) MeV/nucleon. (a) s (atomic mass = 31.97207 u) (b) 2U (atomic mass = 235.04393 u) (Atomic masses: proton (H = 1.00728 u; neutron (¿n) = 1.00867 u; electron (-je) = 0.00055 u; 1 u = 1.6605 x 10-2" kg; speed of light, c = 3.00 x 10 m/s; 1 MeV = 1.602 x 10-13 J) 7.arrow_forwardcentral maximum. (b) Say two things that would make width of the central maximum smaller? Why do some nuclides (like 1) decay? (b) What type nuclide will always decay?arrow_forwardThe radionuclide 208/81 I1 is the daughter nuclide resulting from the a decay of what 208 Tl after 81 parent nuclide? In other words, what isotope would give rise to emitting an a particle?arrow_forward
- The experimentally measured mass of the 200Hg atom is 199.968316 u. Find the binding energy per nucleon predicted by the semiempirical binding energy formula and compare with the actual value of e for this nucleusarrow_forwardEnergy input is required to fuse medium-mass nuclei, such as iron or cobalt, into more massive nuclei. Explain whyarrow_forwardFind question in Image.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios