
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
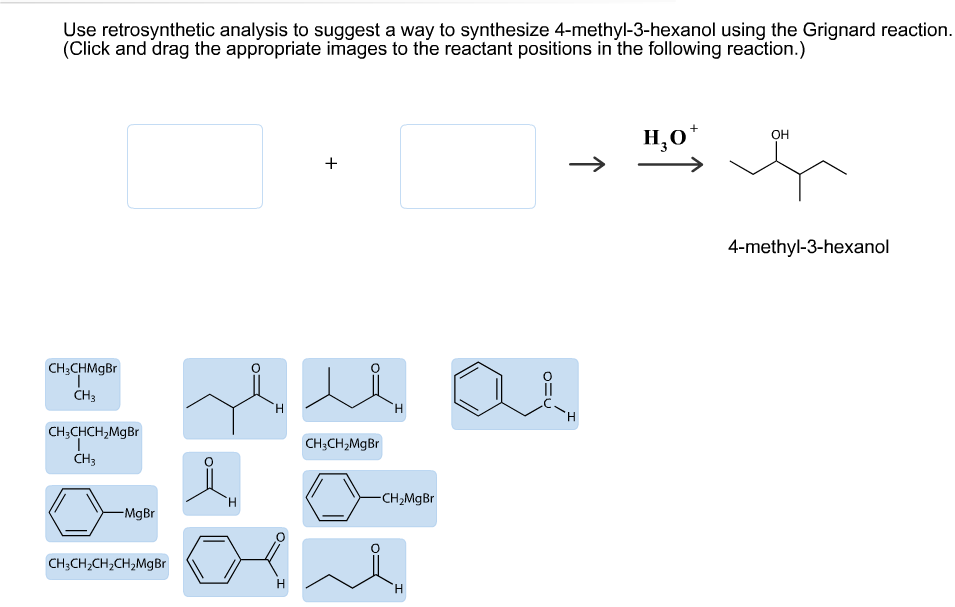
Transcribed Image Text:Use retrosynthetic analysis to suggest a way to synthesize 4-methyl-3-hexanol using the Grignard reaction
(Click and drag the appropriate images to the reactant positions in the following reaction.)
Н,о*
ОН
4-methyl-3-hexanol
CH3CHMgBr
CH3
Н
CH3CHCH2MgBr
CH3CH2MgBr
CH3
CH2Mg Br
H
-Mg Br
CH3CH2CH2CH2Mg Br
Н
"Н
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps with 4 images

Knowledge Booster
Similar questions
- After each arrow fill in the major product of the reactions below. Assume that there is one molar equivalent of each of the reagents unless otherwise stated. ດ Hacoli OCH₂ H300 NaOCH 3 CH₂OH o 12/1 SOH 2) CH3CH2NH, HNOS 2504 00 い 十 Na bet EtOH NH (equivalent) weak base (Nazlos) я NaCN, HCL 1) LDA, -78°C 2)arrow_forwardThe following sequence of steps converts (R)-2-octanol to (S)-2-octanol. ОН ОН p-TSCI CH,COO Na+ A 1. LIAIH, pyridine DMSO 2. H,О (R)-2-Octanol (S)-2-Octanol Propose structural formulas for intermediates A and B, specify the configuration of each, and account for the inversion of configuration in this sequence.arrow_forwardAmong the tertiary alcohols shown below, which one cannot be synthesized by reacting an appropriate Grignard reagent with an ester? CH2CH3 H3C-C-OH CH3 CH3 H3C-C-OH CH3 CH2CH3 H3C-C-OH CH2CH3 CH,CH3 H3C-C-OH CH(CH3)2 CH(CH3)2 H3C-C-OH CH(CH3)2arrow_forward
- Please don't provide handwriting solutionarrow_forwardComplete the following reaction diagram by giving either the reaction conditions or the missing products. The reaction mechanisms are not required.arrow_forward15) In the reaction below, identify the starting material (1) A 1) NH3/HCN 2) HCI/H₂O OH B CH3 H₂N-CH- C -COOH Darrow_forward
- 12. Predict the product(s) for the following reaction. CH₂OC(CH2)14CH3 NaOH excess CHOC(CH2)16CH 3 О CH₂OC(CH2)14CH3 Δarrow_forward6. Write the mechanism for the base-catalyzed keto-enol interconversion shown below. H₂C CH3 OH CH2=CH—C—CH3 H₂C OH CH3 7. Write a mechanism for the following reaction: + CH3–CH2CH,—C-H OH A CH₂CH3arrow_forwardIndicate the product of Nucleophilic Substitution and Elimination of the following reactions(Note: draw proper settings when needed)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY