
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
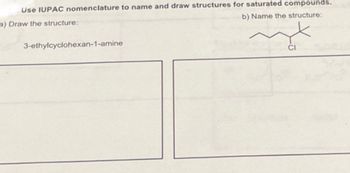
Transcribed Image Text:Use IUPAC nomenclature to name and draw structures for saturated compounds.
b) Name the structure:
a) Draw the structure:
3-ethylcyclohexan-1-amine
CI
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 4 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Consider each of the following organic molecules. Where a structural formula is provided, supply the correct name for the compound, using IUPAC systematic nomenclature. If a name is provided, draw a correct structural formula using bond-line formula notation. fut CH 2,2-dimethyl-1-aminopentane 2-heptanone 1-fluoro-4-iodocyclooctane 2,7-dibromo-1-chloro-4-propyloctanearrow_forward1. Draw structure of the following compounds? A) ethyldimethylamine B) N,N-dimethylbutanamide C) 2-chlorobutanoic acidarrow_forwardDraw the skeletal structure of butan-1-amine from the condensed formula (shown below). CH3CH2CH2CH2NH2arrow_forward
- 4) Using structural diagrams, write balanced equations showing the following reactions. State the type of reaction that is occurring in each question and name each product that is formed. a) The reaction of methanol and oxygen gas. b) The reaction of 2-butene and hydrogen gas c) The reaction between 1-propanol and ethanoic acid d) The reaction between 1-amino-2-methyl pentane and propanoic acidarrow_forwardHow do you draw the structural formula for 2,4,6-trimethylpyridine?arrow_forwardWhich statements are true about amines? NH3+ is the form found in our blood amines can react with aldehydes to produce ethers amines act as weak acids in aqueous solutions amines can react with carboxylic acid to produce alcoholsarrow_forward
- Draw out an appropriate chemical structure for the name provided. m-methylanilinearrow_forwardQuestion 17 of 20 What is the major structural difference between PGE and PGF? A) PGE has a carboxylic acid group and PGF does not. B) PGE has no carbon=carbon double bonds, while PGF does. C) PGE has a ketone group on carbon 9, while PGF has an alcohol group at the same location. D) PGE has a 5-membered carbon ring, while PGF has a 6-membered carbon ring. E) PGE has no alcohol groups, while PGF does.arrow_forwardHow does a soap's dirt removal mechanism work? Describe the structure of the soap by drawing.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY