
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
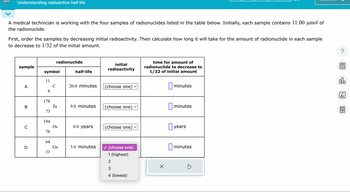
Transcribed Image Text:Understanding radioactive half life
A medical technician is working with the four samples of radionuclides listed in the table below. Initially, each sample contains 11.00 µmol of
the radionuclide.
First, order the samples by decreasing initial radioactivity. Then calculate how long it will take for the amount of radionuclide in each sample
to decrease to 1/32 of the initial amount.
radionuclide
sample
initial
radioactivity
symbol
half-life
time for amount of
radionuclide to decrease to
1/32 of initial amount
11
A
20.0 minutes
(choose one)
☐ minutes
6
178
B
Ta
9.0 minutes
(choose one)
☐ minutes
73
194
C
Os
6.0 years
(choose one)
☐ years
76
64
D
Ga
3.0 minutes
(choose one)
☐ minutes
31
1 (highest)
2
☑
3
4 (lowest)
000
18
Ar
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- A medic hnican is working with the four samples of radionuclides listed in the table below. Initially, each sample contains 22.00 umol of the radionuclide. First, order the samples by decreasing initial radioactivity. Then calculate how long it will take for the amount of radionudlide in each sample to decrease to 1/8 of the initial amount. radionuclide time for amount of initial radioactivity radionuclide to decrease to 1/8 of initial amount sample symbol half-life 139 Ce 58 138. days (choose one) V days A 125 60.0 days (choose one) v O days B 53 77 Br 35 57.0 hours |(choose one) v hours 51 Mn O minutes D 46.0 minutes [choose one) v 25arrow_forwardA medical technician is working with the four samples of radionuclides listed in the table below. Initially, each sample contains 24.00 umol of the radionuclide. First, order the samples by decreasing initial radioactivity. Then calculate how long it will take for the amount of radionuclide in each sample to decrease to 1/3 of the initial amount. radionuclide time for amount of radionuclide to decrease to 1/32 of initial amount sample Initial symbol half-ife radioactivity 177 Lu 71 7.0 days tehnose one 0 O days 212 Bi O nours 1. hour (choose one) e 83 85 O days Sr 65.0 days (choosa one) B 38 88 107. days O days D. Y (choose one) B 39 time for amount of radionuclide to decrease to radionuclide Initial sample radioactivity symbol half-life 1/32 of initial amount 177 Lu 7.0 days O days A v (choose one) 71 1 (highest) 212 Bi 83 1. hour O hours 3. 4 (lowest) 85 Sr 38 I days 65.0 days (choose one) O 88 107. days O days D Y. (choose one) B 39arrow_forwardA medical technician is working with the four samples of radionuclides listed in the table below. Initially, each sample contains 22.00 umol of the radionuclide. First, order the samples by decreasing initial radioactivity. Then calculate how long it will take for the amount of radionuclide in each sample to decrease to 1/4 of the initial amount. time for amount of initial radioactivity radionuclide to decrease to 1/4 of initial amount radionuclide sample symbol half-life 51 Cr 28. days (choose one) v days A 24 179 Ta 2. years (choose one) v years B 73 201 T1 73.0 hours (choose one) v hours 81 46 Sc 84.0 days (choose one) v O days D 21 (choose one) 1 (highest) 4 (lowest)arrow_forward
- First order the samples by decreasing initial video activity then calculate how long it will take for the amount of radionuclides in each sample to decrease to 1/8 of the initial amountarrow_forwardFluorine-18, which has a half-life of 110 minmin, is used in PET scans. 2) If 120. mgmg of fluorine-18 is shipped at 7:00 A.M., how many milligrams of the radioisotope are still active when the sample arrives at the radiology laboratory at 4:10 P.M.?arrow_forward* → esc → C 1 O NUCLEAR CHEMISTRY Understanding radioactive half life sample 1 6 A B Explanation A Announcemer X С A medical technician is working with the four samples of radionuclides listed in the table below. Initially, each sample contains 14.00 umol of the radionuclide. First, order the samples by decreasing initial radioactivity. Then calculate how long it will take for the amount of radionuclide in each sample to decrease to 1/32 of the initial amount. D Q ? N symbol 90 39 55 @ 27 133 54 203 82 Y 2 radionuclide Co https://www-awu.aleks.com/alekscgi/x/Isl.exe/10_u-IgNslkr7j8P3jH-IBMBkpcnaFu0F7UjsroJMKrbFHXPNvwILIE9DNBkbkvSm.... A Xe Pb 124 * Check S W X ALEKS-Chiso X alt # [tx half-life 64.0 hours 18. hours 5.0 days 2.0 days 3 * Q Search E D $ C 4 amathxl pearso X initial radioactivity (choose one): (choose one) v 14-jad (choose one) (choose one) ✓ R F 0 % V do 5 T G time for amount of radionuclide to decrease to 1/32 of initial amount 14- ^ Homework an X 6 B Y H hours hours…arrow_forward
- A medical technician is working with the four samples of radionuclides listed in the table below. Initially, each sample contains 17.00 µmol of the radionuclide. First, order the samples by decreasing initial radioactivity. Then calculate how long it will take for the amount of radionuclide in each sample to decrease to 1/32 of the initial amount. 000 radionuclide sample initial radioactivity time for amount of radionuclide to decrease to 1/32 of initial amount Ar days A days B hours C hours D symbol 192 Ir 77 188 74 62 30 67 31 W Zn Ga half-life 74.0 days 69.0 days 9.0 hours 78.0 hours (choose one (choose one) (choose one) (choose one) X ?arrow_forwardA medical technician is working with the four samples of radionuclides listed in the table below. Initially, each sample contains 25.00 umol of the radionuclide. First, order the samples by decreasing initial radioactivity. Then calculate how long it will take for the amount of radionuclide in each sample to decrease to 1/4 of the initial amount. radionuclide time for amount of radionuclide to decrease to 1/4 of initial amount initial radioactivity olo sample symbol half-life 64 Ga 31 3. minutes v (choose one) 1 (highest) Iminutes A 203 3 2. days I days Pb 4 (lowest) 82 122 4. minutes (choose one) minutes 53 32 14. days I days D (choose one) 15 Explanation Check Privacy Accessibility 2021 McGraw-Hill Education. All Rights Reserved. Terms of Use TO W MAY 1 10 MacBook Airarrow_forwardNonearrow_forward
- A medical technican is working with the four samples of radionuclides listed in the table below. Initially, each sample contains 6.00 umol of the radionuclide. First, order the samples by decreasing initial radioactivity. Then calculate how long it will take for the amount of radionudide in each sample to decrease to 1/4 of the initial amount.arrow_forwardA medical technician is working with the four samples of radionuclides listed in the table below. Initially, each sample contains 13.00 µmol of the radionuclide. First, order the samples by decreasing initial radioactivity. Then calculate how long it will take for the amount of radionuclide in each sample to decrease to 1/16 of the initial amount. radionuclide sample initial radioactivity symbol half-life 46 A Sc 84.0 days (choose one) 21 21 time for amount of radionuclide to decrease to 1/16 of initial amount ☐ days 91 B Y 59.0 days (choose one) ☐ days 39 ur 52 C Mn 6.0 days (choose one) ☐ days 25 18 D F 2. hours (choose one) hours 9 1 (highest) half 2 3 4 (lowest) х Ar ?arrow_forwardoudos A medical technician is working with the four samples of radionuclides listed in the table below. Initially, each sample contains 11.00 umol of the radionuclide. First, order the samples by decreasing initial radioactivity. Then calculate how long it will take for the amount of radionuclide in each sample to decrease to 1/16 of the initial amount.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY