
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question

Transcribed Image Text:Under standard conditions, the galvanic cell shown above has a cell potential of+1.56 V using the reaction given. The salt bridge contains KNO3, which allows K ions and NO, ions to move in
the directions indicated. If KNO3 in the salt bridge is replaced with KOH, some Zn(OH), (s) precipitates in the Zn-Zn(NO,), half-cell. Which of the following best explains how the cell potential
is affected as Zn(OH), (s) starts to precipitate, and why?
The cell potential increases because the concentration of Zn2+(ag) decreases and Q.
,becomes smaller.
|Ag"
The cell potential decreases because the concentration of Zn2+ (ag) decreases and Q,
Zn
becomes smaller.
(Ag
The cell potential increases because Kt ions replace Zn2+ ions and the reduction of K* is more thermodynamically favored than the reduction of Zn?+.
D
The cell potential stays the same because Zn(OH), (8) is not part of the redox reaction responsible for the operation of the galvanic cell.
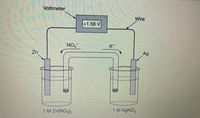
Transcribed Image Text:Voltmeter
Wire
+1.56 V
K+
NO3
Ag
Zn
1 M AgNO3
1 M Zn(NO3)2
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Consider an electrochemical cell with the following cell notation: Fe(s)| Fe²+ (aq) (from saturated FeCO3) || Fe²+ (aq) (0.150 M) | Fe(s) Given: Ksp of FeCO3 is 3.5 x 10-11 Eºred Fe²+/Fe = -0.44V Which of the following is the expected oxidation half-reaction based on the given cell notation? O FeCO3(s) Fe²+ (aq) + CO3²- (aq) O Fe²+ (aq) + CO3²- (aq) =1 FeCO3(s) O Fe2+ *(aq) + 2 e¯ = Fe(s) O Fe(s) Fe²+ *(aq) + 2 e-arrow_forwardA standard galvanic cell is constructed in which a Co2+ | Co half cell acts as the cathode. Which of the following statements are correct? Hint: Refer to a table of standard reduction potentials. (Choose all that apply.) The cathode reaction is Co2+ + 2e -> Co. Co is oxidized at the cathode. The anode compartment could be Fe³+|Fe²+. As the cell runs, anions will migrate from the Co²+ | Co compartment to the other compartment. In the external circuit, electrons flow from the Co²+ | Co compartment to the other compartment.arrow_forwardConsider a galvanic cell with the anode containing a Zn metal electrode and a 0.10M Zn(NO3)2 solution and the other half-cell containing a Sn metal electrode and a 0.10M Sn(NO3)2 solution. If the measured Ecell value is +650mV and the Zn2+/Zn reduction potential is assumed to be -790mV, what is the Sn2+/Sn reduction potential?arrow_forward
- 5) For this problem refer to the electrochemical cell below a) Which electrode is the cathode and which is the anode? b) Is this cell electrolytic or galvanic? Explain. c) If the concentration of dissolved zinc ions were increased, how would this change the measured voltage? Volumeter so so; Znisi - Zntag) - 2e Cagl + 2e -Cuis)arrow_forwardHow long will it take in hours to create 1.026 grams of Cr at the cathode of an electrolytic cell if 265.8 mA of current is passed through a solution containing Cr(NO3)6 molar mass of Cr is 51.9901arrow_forwardA standard galvanic cell is constructed in which a Pb2+ | Pb half cell acts as the cathode. Which of the following statements are correct? Hint: Refer to a table of standard reduction potentials. (Choose all that apply.) The anode compartment could be I2|I-. The cathode reaction is Pb2+ + 2e- -> Pb Pb is oxidized at the cathode. Pb2+ is reduced at the cathode. In the external circuit, electrons flow from the other compartment to the Pb2+|Pb compartment.arrow_forward
- A standard galvanic cell is constructed in which a Cd²+ | Cd half cell acts as the cathode. Which of the following statements are correct? Hint: Refer to a table of standard reduction potentials. (Choose all that apply.) In the external circuit, electrons flow from the other compartment to the Cd2"|Cd compartment. The cathode reaction is Cd -> Cd²+ + 2e¯ Br2|Br could be the other standard half cell. U Cr**|Cr2+ could be the other standard half cell. The cathode reaction is Cd2+ + 2e -> Cdarrow_forwardA standard galvanic cell is constructed in which a Cd²+ | Cd half cell acts as the cathode. Which of the following statements are correct? Hint: Refer to a table of standard reduction potentials. (Choose all that apply.) O Cd is oxidized at the cathode. Zn2"|Zn could be the other standard half cell. Hg-"Hg could be the other standard half cell. As the cell runs, anions will migrate from the other compartment to the Cd-"|Cd compartment. In the external circuit, electrons flow from the other compartment to the Cd²"|Cd compartment.arrow_forwardA standard galvanic cell is constructed with Mg2+ | Mg and Br₂ Br half cell compartments connected by a salt bridge. Which of the following statements Hint: Refer to a table of standard reduction potentials. (Choose all that apply.) O The cathode compartment is the Br₂ | Br compartment. The anode compartment is the Br₂ Br compartment. In the external circuit, electrons flow from the Mg2+ | Mg compartment to the Br₂ | Br compartment. As the cell runs, anions will migrate from the Mg2+ | Mg compartment to the Br₂ | Br compartment. O Mg2+ is reduced at the cathode.arrow_forward
- t/takeCovalentActivity.do?locator-assignment-take [Review Topics] [References] Use the References to access important values if needed for this question. A standard galvanic cell is constructed in which a Pb2+ | Pb half cell acts as the cathode. Which of the following statements are correct? Hint: Refer to a table of standard reduction potentials. (Choose all that apply.) Fe3+|Fe2+ could be the other standard half cell. Mn2+1Mn could be the other standard half cell. OPb is oxidized at the cathode. 0 The cathode reaction is Pb2+ + 2e -> Pb As the cell runs, anions will migrate from the Pb2+|Pb compartment to the other compartment. Submit Answer Retry Entire Group 7 more group attempts remainingarrow_forwardA standard galvanic cell is constructed in which a Cu2+ | Cu+ half cell acts as the cathode. Which of the following statements are correct? Hint: Refer to a table of standard reduction potentials. (Choose all that apply.) As the cell runs, anions will migrate from the other compartment to the Cu2+|Cu+ compartment. The cathode reaction is Cu+ -> Cu2+ + e-. F2|F- could be the other standard half cell. In the external circuit, electrons flow from the other compartment to the Cu2+|Cu+ compartment. The anode reaction could be Zn -> Zn2+ + 2e-.arrow_forwardUse the References to access important values if needed for this question. A standard galvanic cell is constructed in which a Cu²* | Cu half cell acts as the cathode. Which of the following statements are correct? Hint: Refer to a table of standard reduction potentials. (Choose all that apply.) O Cu is oxidized at the cathode. O Cr3+|Cr2+ could be the other standard half cell. The anode reaction could be Cr2* -> Cr³* + e Ag"Ag could be the other standard half cell. U As the cell runs, anions will migrate from the Cu2"|Cu compartment to the other compartment. Submit Answer Try Another Version 2 item attempts remainingarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY