
Human Anatomy & Physiology (11th Edition)
11th Edition
ISBN: 9780134580999
Author: Elaine N. Marieb, Katja N. Hoehn
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
I need assistance with the table. Which contains peptide bonds

Transcribed Image Text:C42
5. Name the elements found in carbohydrates: Crbon hydrorem.oxren
Name the elements found in fats: Car bon hudrogencxgen
How do fats differ from carbohydrates with respect to the elements that comprise them?
Fats contain a Sienificantly lococramount of
22-44 84,
Compeared to carbohgdtrates.
Oxyger
114
6. Give the range of the pH scale:
Differentiate between acids and bases. (Include H+ concentration and pH values)
An Gcidic pH balance is betueen 1-6 and a basic concentration 13 b
The laver the pH, the hugher the hydrogen ion concentrathon is.
7. Complete the table below from the choices on the back
letter
statement
contains peptide bonds
part of the molecule forms the hydrophobic part of cell
membranes
contains 1-4 and 1-6 glycosidic bonds
forms the primary structure of a protein
H.
used for energy storage in plants
forms a helical structure
the sub-unit molecule is B-glucose
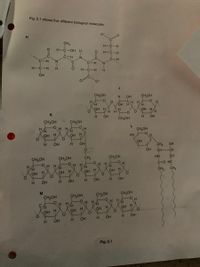
Transcribed Image Text:Fig. 5.1 shows five different biological molecules.
H
CH3
H-
H-C-OH
H
H-C-H
C-H
.C
C-H
CH
N.
N.
C.
C-H
H-C-H
H.
H-C-H
H.
OH
CH,OH
H
OH
CH,OH
H
H.
OH H
ОН Н
OH
H
H
-C
H
OH
OH
CH,OH
CH,OH
CH,OH
H.
H.
CH,OH
OH
OH H
HO
H
OH
H
OH
OH
CH2
OH
OH
CH
CH
CH,OH
CH2OH
CH2
CH,OH
HN
CH
H.
H.
H.
C=0 HC
H.
H.
OH H
H.
H
H.
OH
Н
CH2 CH2
H.
OH
H.
OH
OH
H.
CH,OH
CH,OH
CH,OH
CH,OH
нн
H.
H/
OH H
H
OH H
OH
OH H
H
OH
H.
OH
H.
OH
Fig. 5.1
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biology and related others by exploring similar questions and additional content below.Similar questions
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Human Anatomy & Physiology (11th Edition)BiologyISBN:9780134580999Author:Elaine N. Marieb, Katja N. HoehnPublisher:PEARSON
Human Anatomy & Physiology (11th Edition)BiologyISBN:9780134580999Author:Elaine N. Marieb, Katja N. HoehnPublisher:PEARSON Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax
Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax Anatomy & PhysiologyBiologyISBN:9781259398629Author:McKinley, Michael P., O'loughlin, Valerie Dean, Bidle, Theresa StouterPublisher:Mcgraw Hill Education,
Anatomy & PhysiologyBiologyISBN:9781259398629Author:McKinley, Michael P., O'loughlin, Valerie Dean, Bidle, Theresa StouterPublisher:Mcgraw Hill Education, Molecular Biology of the Cell (Sixth Edition)BiologyISBN:9780815344322Author:Bruce Alberts, Alexander D. Johnson, Julian Lewis, David Morgan, Martin Raff, Keith Roberts, Peter WalterPublisher:W. W. Norton & Company
Molecular Biology of the Cell (Sixth Edition)BiologyISBN:9780815344322Author:Bruce Alberts, Alexander D. Johnson, Julian Lewis, David Morgan, Martin Raff, Keith Roberts, Peter WalterPublisher:W. W. Norton & Company Laboratory Manual For Human Anatomy & PhysiologyBiologyISBN:9781260159363Author:Martin, Terry R., Prentice-craver, CynthiaPublisher:McGraw-Hill Publishing Co.
Laboratory Manual For Human Anatomy & PhysiologyBiologyISBN:9781260159363Author:Martin, Terry R., Prentice-craver, CynthiaPublisher:McGraw-Hill Publishing Co. Inquiry Into Life (16th Edition)BiologyISBN:9781260231700Author:Sylvia S. Mader, Michael WindelspechtPublisher:McGraw Hill Education
Inquiry Into Life (16th Edition)BiologyISBN:9781260231700Author:Sylvia S. Mader, Michael WindelspechtPublisher:McGraw Hill Education

Human Anatomy & Physiology (11th Edition)
Biology
ISBN:9780134580999
Author:Elaine N. Marieb, Katja N. Hoehn
Publisher:PEARSON

Biology 2e
Biology
ISBN:9781947172517
Author:Matthew Douglas, Jung Choi, Mary Ann Clark
Publisher:OpenStax

Anatomy & Physiology
Biology
ISBN:9781259398629
Author:McKinley, Michael P., O'loughlin, Valerie Dean, Bidle, Theresa Stouter
Publisher:Mcgraw Hill Education,

Molecular Biology of the Cell (Sixth Edition)
Biology
ISBN:9780815344322
Author:Bruce Alberts, Alexander D. Johnson, Julian Lewis, David Morgan, Martin Raff, Keith Roberts, Peter Walter
Publisher:W. W. Norton & Company

Laboratory Manual For Human Anatomy & Physiology
Biology
ISBN:9781260159363
Author:Martin, Terry R., Prentice-craver, Cynthia
Publisher:McGraw-Hill Publishing Co.

Inquiry Into Life (16th Edition)
Biology
ISBN:9781260231700
Author:Sylvia S. Mader, Michael Windelspecht
Publisher:McGraw Hill Education