
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
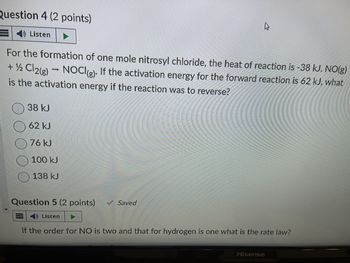
Transcribed Image Text:Question 4 (2 points)
Listen
For the formation of one mole nitrosyl chloride, the heat of reaction is -38 kJ. NO(g)
+ 1/2 Cl2(g) → NOCI(g). If the activation energy for the forward reaction is 62 kJ, what
is the activation energy if the reaction was to reverse?
38 kJ
62 kJ
76 kJ
100 kJ
138 kJ
Question 5 (2 points)
Listen
✓ Saved
If the order for NO is two and that for hydrogen is one what is the rate law?
Hisense
Expert Solution
arrow_forward
Step 1

Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- If an increase of 10 K in temperature doubles the rate constant of a reaction. What must the activation energy (in kJ/mol) be if a temperature increased 10 degrees celsius from 54.4 ° C? Note: if one of the options is "INF", it means infinity R = 8.3145 J/K.mol The units for all the options below is kJ/mol Select one: O a. 63.7 Ob. 19.8 Oc. INF Od. 25.4 O e. 127.3 Clear my choicearrow_forwardWhat is the activation energy of a reaction that is three times faster at 40.0 °C than it is at 20.0 °C? ***Answer key: 41.93 kJ/molarrow_forwardQuestion 8 of 11 Submit The activation energy, Ea, for a particular reaction is 13.6 kJ/mol. If the rate constant at 475 K is 0.0450 1/min, then what is the value of the rate constant at 737 K? (R = 8.314 J/mol • K) | 1/min 1 2 3 4 6. C 7 8 +/- x 10 0 Tap here or pull up for additional resources LOarrow_forward
- # 3 E D C Energy diagrams for two reactions are shown. Macmillan Learning Energy (kJ/mol) e 150 100 50- What is the heat of reaction for Reaction A? AHrxnA = 4 FER 20 What is the activation energy for Reaction A? R F V Reaction progress Reaction A 5 T G Search or type URL G B tv 6 MacBook Pro Y Ⓒ H 201 7 N U S J kJ mol 00 8 M I Energy (kJ/mol) 150 T 100- NIMZAO ✪ 50 What is the heat of reaction for Reaction B? AHxnB = What is the activation energy for Reaction B? K ( - O 9 < O I * ) -C O Reaction progress Reaction B L command ») P . V = : P W ; 242) { option [ SEC e = ? O kJ mol 1 delete retuarrow_forwardEnergy diagrams for two reactions are shown. 150 150 100 100 50- 50- Reaction progress Reaction progress Reaction A Reaction B What is the heat of reaction for Reaction A? What is the heat of reaction for Reaction B? AHrxn A kJ AHrxn B kJ mol mol What is the activation energy for Reaction A? What is the activation energy for Reaction B? kJ kJ Ea rxn A = Ea rxn B = mol mol Energy (kJ/mol)arrow_forwardThe energy of activation for the decomposition of 2 mol of HI to H2 and I2 in the gas phase is 185 kJkJ. The heat of formation of HI(g) from H2(g) and I2(g) is -5.65 kJ/mol Part A Find the energy of activation for the reaction of 1 mol of H2 and 1 mol of I2 in the gas phase.arrow_forward
- + Twychoad Energy (kJ/mol) -50 +37.5 25 12.5 Based on the reaction energy diagram provided, which of the following statements is incorrect (false)? Reaction Profile Question 15 of 15 3 A) The activation energy is 12.5 kJ/mol. B) The energy change (AE or AH or AG) for the reaction is 25 kJ/mol. C) The reaction is exothermic. D) The reactants are at the position labeled 1. E) The products are at the position labeled 3. Submitarrow_forward6:47 PM Sun Feb 5 The activation energy for a particular reaction is 102 kJ/mol. If the rate constant is 1.35 x 104 s¹ at 305 K, what is the rate constant at 273 K? Tap here or pull up for additional resources 2 2 #3 $ 4 → Question 41 of 50 % < 60 & 7 8arrow_forwardQuestion 2 of 19 A substance decomposes with a rate constant of 9.05 x 104 s¹. How long does it take for 14.5% of the substance to decompose? Tap here or pull up for additional resources MacBook Air 1 2 4 5 7 8 +/- 3 6 9 0 S Submit x с x 100arrow_forward
- 7. Reactions A and B were studied and found to produce the given energy diagrams. 150 150 Energy 100 (kJ/mol) 100 Energy (kJ/mol) 50 50 Reaction progress Reaction progress Reaction A Reaction B A third reaction, C was studied with a measured activation energy of 55 KJ/mol. Assume all reactions take place at the same temperature. Reaction A is slowest a. b. Reaction B is slowest c. Reaction C is slowest d. All reactions happen at the same rate.arrow_forwardWhat is the activation energy of the forward reaction? Question 15 options: 200 kJ 150 kJ 250 kJ 100 kJarrow_forwardgive typed answer plzz no handwrittenarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY