
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
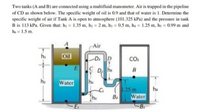
Transcribed Image Text:Two tanks (A and B) are connected using a multifluid manometer. Air is trapped in the pipeline
of CD as shown below. The specific weight of oil is 0.9 and that of water is 1. Determine the
specific weight of air if Tank A is open to atmosphere (101.325 kPa) and the pressure in tank
B is 113 kPa. Given that: hị = 1.35 m, hz = 2 m, hy = 0.5 m, hạ = 1.25 m, hs = 0.99 m and
he = 1.5 m.
Air
h:
Oil
D:
D
CO:
D. h.
B
h:
Water
he
25 m
he
the
Water
B:
E:
B:
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- A Q4: A diffuser, has air entering at 100 kPa, 280 K, with a velocity of 200 m/s. The inlet cross-sectional area of the diffuser is 100 mm2. At the exit, the area is 860 mm2, and the exit velocity is 20 m/s. Determine the exit pressure and temperature of the air. Take Cp=1.005 KJ/kg.K A: 280 K B: 300 K C: 320 K D: 340 K * 2) Determine the exit pressure of the air if inlet temperature of the air 300 K. A: 124 KP B: 134 KP C: 144 KP * D: 154 KParrow_forward1. A hole in the bottom of a large open tank discharges water to the atmosphere. If the exit velocity in the absence of losses is Ve, find the loss coefficient for the hole if the actual velocity is Ve/2. Assume turbulent flow. 2. Air enters a duct at a speed of 100 m/s and leaves it at 200 m/s. If no heat is added to the air and no work is done by the air, what is the change in temperature of the air as it passes through the duct?arrow_forward8arrow_forward
- please show how to derive h using nusselt equation and solve for qheatlossarrow_forwardA 7 dm3 rigid tank contains 4.5 kg of saturated water (liquid and vapor) at 38ºC. The tank is slowly heated. a. After a long period of time what do you think happened to the liquid water surface: do you think it raised and reached the top of the tank or it dropped and reached the bottom of it? b. Solve the same problem considering that the amount of water is 0.45 kg instead.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The