Question
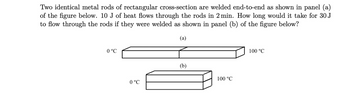
Transcribed Image Text:Two identical metal rods of rectangular cross-section are welded end-to-end as shown in panel (a)
of the figure below. 10 J of heat flows through the rods in 2 min. How long would it take for 30 J
to flow through the rods if they were welded as shown in panel (b) of the figure below?
(a)
0 °C
0 °C
(b)
100 °C
100 °C
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps

Knowledge Booster
Similar questions
- how to solve this Using section B, calculate the amount of ice used to produce the graph Use section C of the graph above to determine the amount of ice used to produce the graph.arrow_forwardQUESTION 9 During the video on heat conduction, H through a single pane window was calculated. Now, consider a window that has wo panes with a 6 mm air gap between them. Each pane has an area of 1.9 m. The window separates the inside of a house at 22 °C from the outside at 0°C. Let Kair = 0.03 W/(m-K). Hint: This problem can be simplified by noting that the thermal conductivity of glass is much greater than air, so the effect of the glass on the total conductivity can be ignored. Therefore, the purpose of the two glass panes is to support the layer of air between them. Calculate the heat current H through the double glass pane window in watts. Do not include units in your answer and state your numerical answer in normal form. QUESTION 10 Consider process 3 in the figure below (or in First_Exam_Figures.pdf) shown by the bold solid line with an arrowhead. The pressure of a gas is decreased at constant volume. V. Click Save and Submit to save and submit. Click Save All Ansuers to save all…arrow_forwardBased from the graph of Temperature versus Energy as shown, which of the following statements is true? * T (°C) D 120 90 60 30 I O I C B O Latent heat of fusion is present in portion B only. O Latent heat of fusion is present in portions A and B. O Latent heat of condensation is present in portion D only. O Latent heat of vaporization is present in portions C & D. Earrow_forward
- 1000 800 600 400 200 10 20 30 40 50 60 70 80 90 -200 400 Time in seconds Look at the graph above. How much did the temperature change from t = 30 to t = 60 seconds? %3D AT = unit Select an answer v This substance has a specific heat of 0.13 cal/g°C. If you have 34 grams of this substance, how much heat was added from t = 30 to t = 60 seconds? Q = unit Select an answer v Temperature In Carrow_forwardI need help with this questionarrow_forwardYou must use the Method of Mixtures to solve the problem. You must state which substances lose heat and which gain heat at the start of your work. Twelve ounces of copper at 300 degrees Fahrenheit and four ounces of zinc at 200 degrees Fahrenheit are placed in a brass container of water. The eight-ounce brass container is at 50 degrees Fahrenheit. The water is also at 50 degrees Fahrenheit. The final temperature of the mixture is 62.63736264 degrees Fahrenheit. The specific heat of copper is 0.092 Btu/lb oF The specific heat of zinc is 0.092 Btu/lb oF The specific heat of brass is 0.092 Btu/lb oF The specific heat of (liquid) water is 1.00 Btu/lb oFWhat amount of water was in the brass container? No round off until final answerarrow_forward
- When a 270-g piece of iron at 160 °C is placed in a 95-g aluminum calorimeter cup containing 250 g of liquid at 10° C, the final temperature is observed to be 35 °C. The value of specific heat for iron is 450 J/kg·C°, and for aluminum is 900 J/kg · C°. Part A Determine the specific heat of the liquid. Express your answer using two significant figures. VO AE ? J C = kg-C° Submit Request Answerarrow_forwardHow much energy is required to raise the temperature of 0.2 kg of water from 120.°C to 80.°C? Answer in J. (What equation/ method should I use to solve this? Must I also convert the temperature to Kelvin if I'm not mistaken?)arrow_forwardTemperature & Heat 13. At 30 °C the volume of an aluminum sphere is 30 cm³. The coefficient of linear expansion is 24 x 10 C¹. If the final volume is 30.5 cm³, what is the final temperature of the aluminum sphere? Assume B = 3 a 14. A 500-gram cube of lead is heated from 25 °C to 75 °C. How much energy was required to heat the lead? The specific heat of lead is 0.129 J/g-°C. 15. How much heat is required to convert 135 g of ice at -15 °C into water vapor at 120 °C? (Assume c of steam = 1.996 J/g-°C, c of water = 4.1858 J/g-°C, c of ice =2.090 J/g-°C. Lv of water = 540 cal/g & Lf of water = 80 cal/g)arrow_forward
- I need typing clear urjentarrow_forward⦁ When a certain quantity of helium gas contained inside and engine cylinder combusts, the expanding gas causes the 12-cm-radius piston to move outward by 10.0 cm. If the gas remains at a pressure of 120 kPa for this process, what is the heat transfer? (See the diagram at the top of the next page.)⦁ 1,960 J⦁ 1,660 J⦁ 1,360 J⦁ 1,060 J⦁ 760 Jarrow_forward0.4 kg water is at room temperature 23 degrees in an aluminum container. An unknown metal with 0.5 kg mass is placed inside the water raising its temperature to 40 degrees. A)What is the specific heat of the unknown metal. B) Would the equilibrium temperature greater, less or the same if the system was not properly isolated.arrow_forward
arrow_back_ios
arrow_forward_ios