
Biochemistry
9th Edition
ISBN: 9781319114671
Author: Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher: W. H. Freeman
expand_more
expand_more
format_list_bulleted
Question
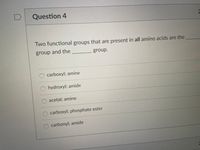
Transcribed Image Text:Question 4
2
Two functional groups that are present in all amino acids are the
group and the
group.
carboxyl; amine
hydroxyl; amide
acetal; amine
carboxyl; phosphate ester
carbonyl; amide
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biochemistry and related others by exploring similar questions and additional content below.Similar questions
- Draw the enantiomer of the monosaccharide by changing the structure below. H ☑ H -ОН H -ОН но -H HO -H CH₂OHarrow_forwardOrganic molecules all contain carbon. Describe the structure and function of each of these organic molecules. carbohydrates, lipids, proteins, nucleic acids.arrow_forwardClassify the chemical consituents that are part of each molecule. Guanosine triphosphate (GTP) three phosphoryl groups two glycines oleate a-D-ribose tyrosine Methionine enkephalin glycerol Answer Bank choline one phosphate Phosphatidylcholine guanine methioninearrow_forward
- The following molecules are lipids. Indicate to which lipid group each belongs.arrow_forwardWhat are the names of the seven functional groups found in organic molecules? Underline the two that can act as an acid. Make the single group that acts as a base in bold lettering.arrow_forwardThe molecule on the left has of 2 ring structures with an O atom in the ring and it ratio of C, H, O is 1:2:1. The molecule on the right has an amino group and a carboxyl group. The structure on the right is a(n) and the structure on the left is a(n) lipid, carbohydrate O amino acid, lipid amino acid, carbohydrate lipid, proteinarrow_forward
- What characteristics do all lipids have in common?arrow_forwardWhat is meant by “polarity” of a polypeptide chain and by “polarity” of a chemical bond? How do the meanings differ?arrow_forwardConsider this monosaccharide. H H OH H .OH HO H HO H CH₂OH Part: 0/3 Part 1 of 3 Label the chirality center(s) for the monosaccharide by highlighting the carbon(s) in the structure below. H H -OH H -OH HO -H HO -H CH₂OH હેarrow_forward
- Which of the following functional groups do NOT contain a carbonyl? Select all that apply. amide ketone O aldehyde etherarrow_forwardThe following is an example of a type of lipid. Answer the following questions based on its structure: OH a. What type of lipid is this categorized as: CH3 b. What two functional groups can be found in this structure? HO Estradiol (estrogen)arrow_forwardWhich of the following organic compound doesnot dissolve in water? glycerol propane propanoic acid propanol H H-C Н Н H-с-с-с Н- OH HICIH H-C c-c 1 Н OH H -+ Н HICIH H Н H H C C H H OH 5-о-т OH -C-H -H OHarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON