
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
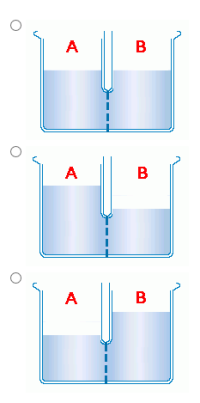
Both pictures are of one problem

Transcribed Image Text:Two aqueous solutions are separated by a semipermeable membrane through which only water
molecules can pass, as shown in the illustration to the left.
A
В
At the start of the experiment, compartment A contains a solution containing 11.7 grams per liter of
the nonelectrolyte ethanol (C2H5OH) and compartment B contains a solution containing 22.8 grams
per liter of the nonelectrolyte glycine (H;NCH2COOH). Which of the following represents the
expected appearance of the apparatus after sufficient time has passed?
11.7 g/L
C;H;OH H2NCH;COOH
22.8 g/L
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- MyCSU - Columbus State Univer. x | № Inbox - bailes_amber@columbu: x D2L Homepage - Columbus State Un X - со A ALEKS-Amber Bail www-awu.aleks.com/alekscgi/x/lsl.exe/1o_u-IgNslkr7j8P3jH-lJgXZp57itWHhRgilODc5MqvhZbKYx2-U-037007TYd Gmail YouTube Maps MyCSU - Columbus... Homepage - Georg... Microsoft Office Ho... B Lesson 2 Disc O CHEMICAL REACTIONS = Solving for a reactant using a chemical equation Ammonia (NH3) chemically reacts with oxygen gas (0₂) to produce nitric oxide (NO) and water (1₂0). What mass of ammonia is consumed by the reaction of 9.9 g of oxygen gas? Be sure your answer has the correct number of significant digits. x10 g ANAKKALE X S ? Email 4 Jessy Vseforestainty a jedan den so $45******arrow_forwardplease answer the last 3 sub-partsarrow_forward1.) PhgP H. F3C Br b.) H. F3C H. F3C Br Br F3C Br d) e) F3C F3C Brarrow_forward
- Chrome File Edit View History Bookmarks People Tab Window Help Hcc Dashbc x E Buy Es: x G find so x © Periodi x A ALEKS X HUc Chapte x E New m x G conver X 不→ C A www-awn.aleks.com/alekscgi/x/lsl.exe/1o_u-IgNsIkr7j8P3jH-IQiHqRdYV_6Ux63SypJXz0Coxvwqgg4JkWI7FgD9QGpr.. O GASES O OC D Jacqueline v Using Avogadro's Law Hydrogen gas and nitrogen gas react to form ammonia gas. What volume of ammonia would be produced by this reaction if 7.5 m of nitrogen were consumed? Also, be sure your answer has a unit symbol, and is rounded to 2 significant digits. 圖 中 ロ alo oloarrow_forward4) The intensity of gamma rays, X-rays, or any other radiation is A) inversely proportional to the cube of the distance from the source. B) inversely proportional to the distance from the source. C) inversely proportional to the square of the distance from the source. D) directly proportional to the square of the distance from the source. E) directly proportional to the distance from the source. dele (1arrow_forwardAnswer question number 7 on the attached documentarrow_forward
- O Course Home b Chemistry Question | bartleby G indivuials with austium good to -> A openvellum.ecollege.com/course.html?courseld=16561285&OpenVellumHMAC=446c877376a9bc7ac878d8ad119f4717#10001 Q * E Apps O Maps E Connect - To Do As... O OCCC Moodle P chem work b help Gmail YouTube Balance Chemical E. II Review | Constants | Periodic Table A carbon atom is chiral if it is bonded to four different groups. For example, CHCIBII is chiral, but CCl,BrI is achiral because some of the bonded groups are the same. If a chiral carbon atom is present, then that molecule has a non-superimposable mirror image called an enantiomer. (Figure 1) Part A Identify all the chiral atoms in the below structure by right-clicking* a chiral atom to bring up a menu that includes "Atom Properties." Click on Atom Properties then click the checked box next to the Map field to clear the checkmark. Then enter "1" in the Map *Mac users: Use an equivalent for right- field box to label that chiral carbon atom. clicking.…arrow_forwardChrome File Edit View History Bookmarks People Tab Window Help * 30% (4) Sat 2:00 PM Q OE o Chem101 O General Chemistry I (LAB SCI) X + i app.101edu.co EApps MM Gmail YouTube 9 Maps A Translate https://www.carth. 9 Google Chrome isn't your default browser Set as default Question 7 of 7 Submit Using the equations 2 Sr(s) + O2 (g) → 2 Sro (s) AH° = -1184 kJ/mol CO2 (g) → C (s) + O2 (g) AH° = 394 kJ/mol kJ/mol Determine the enthalpy for the reaction C(s) + 2 SrO(s) → CO2 (g) + 2 Sr(s). 1 2 6 C 03.0 Se VHFORMA The MO -Standa 8 AH ma +/- x 100 8T國山電@ O etv Oct 24 MacBook Air 80 888 SC FS F6 F3 F2 %23 2$ & delete 3 5 8. W E R Y U H J к K ret F V M 4-arrow_forwardHelp 100% 2 Tue 4: Public Health Ch x * HSC 258 - Major Projec X * Mind Tap - Cengage Lea X C The Illustration To The d=55750828934189288909969212&EISBN=9781305657571&id=1061392007&nbld%3D21... * O TO Q Search this co References Use the References to access important values if needed for this question. For the following reaction, 6.43 grams of sulfur dioxide are mixed with excess oxygen gas . The reaction yields 6.30 grams of sulfur trioxide . sulfur dioxide(g) + oxygen(g) sulfur trioxide(g) grams What is the ideal yield of sulfur trioxide? What is the percent yield for this reaction? Submit Answerarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY