
Elements Of Electromagnetics
7th Edition
ISBN: 9780190698614
Author: Sadiku, Matthew N. O.
Publisher: Oxford University Press
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
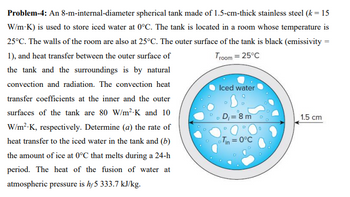
Transcribed Image Text:Troom = 25°C
Problem-4: An 8-m-internal-diameter spherical tank made of 1.5-cm-thick stainless steel (k = 15
W/m-K) is used to store iced water at 0°C. The tank is located in a room whose temperature is
25°C. The walls of the room are also at 25°C. The outer surface of the tank is black (emissivity =
1), and heat transfer between the outer surface of
the tank and the surroundings is by natural
convection and radiation. The convection heat
transfer coefficients at the inner and the outer
surfaces of the tank are 80 W/m²-K and 10
W/m².K, respectively. Determine (a) the rate of
heat transfer to the iced water in the tank and (b)
the amount of ice at 0°C that melts during a 24-h
period. The heat of the fusion of water at
atmospheric pressure is hf5 333.7 kJ/kg.
Iced water
D
D₁= 8 m
Tin = 0°C
1.5 cm
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 5 steps with 16 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Similar questions
- An insulated steam pipe is routed horizontally through an unheated room, then vertically to the ceiling. The pipe is insulated, and the outside diameter of the insulation is 15 cm. The horizontal and vertical lengths of pipe are both 4 m long. The outside-surface temperature of the insulation is 50°C, and the room temperature is 5°C. How much heat is lost from the insulation by convection?arrow_forwardFind the temperature profile of the rectangular plate 4m x 4m, whose temperature at the side are given below: Take delx= del y= 1 m. 100 C 75 C OC 50 Carrow_forwardA thin board of 0.5 cm thickness is exposed to a fluid of T, = 22°C and h = 30 W/m².K from one side with an emissivity of 0.7 where it faces a surrounding whose temperature is Tsur = 30°C. A heat flux of q" = 3500 W/m? is applied to the other side. Each side has an area of 12cm×12cm. Tsur (a) If the board has the following properties: k = 15.1 W/m.K, c, = 480 J/kg.K and p = 8055 kg/m², express an equation for the variation of temperature with respect to time. (b) Find the initial time rate of change of the board temperature if the initial board temperature is q" h 50°C.arrow_forward
- Please solve this problem THank youarrow_forward= A processing plant is convectively heating a solid half-sphere of radius of 12 cm made of pure aluminum (k 237 W/m-K) by placing it on an insulated surface as shown in Fig. 1. It is exposed to hot exhaust gas at 300°C with a convection coefficient of 180 W/m².K. Is the lumped capacitance approximation valid? Figure 1: Half-spherearrow_forwardasaparrow_forward
- A house has a three meter deep basement (foundation) wall that is in a 10 meter by eight meter rectangular shape based on interior dimensions. On average, 2.5 meters of the wall is below grade. Assume the outside air temperature is 38°C and the inside temperature is 20°C; there is an average ground temperature of 27°C. Determine the rate of heat loss through the wall if it is insulated with an R-value of about 30°C.m^2/W.arrow_forwardThe outside temperature on a particular winter's day is 0°C. Consider two identical houses, each of external surface area 200 m² and internal temperature 20°C. The walls of the first are constructed of a single layer of brick, of thermal conductivity 1 W m-1 °C-1 and thickness 20 cm. What is the heat flux Q out of this house? What is its rate of heat loss in Watts? The walls of the second house are constructed of two layers of brick, each 10 cm thick, and a layer of insulation, also 10 cm thick. The insulation is made mostly out of air, thermal conductivity 0.01 W m of heat loss from this house? How different would your answer be if you neglected the layers of brick in this calculation? -1 °C-1. what are the heat flux and the ratearrow_forwardA large spherical storage tank, 2 m in diameter, is buried in the earth at a location where the thermal conductivity is 1.5 W/m.°C. The tank is used for the storage of an ice mixture at 0°C, and the ambient temperature of the earth is 20°C. Calculate the heat loss from the tank.arrow_forward
- I got t=531.96 seconds is that rightarrow_forwardYou are cooling the door to a large heat-treating oven, which is 3.50 m tall by 1.30 m wide, with a large fan blowing air at 25.0°C over the outside of the door. The convection coefficient associated with this cooling process is h = 57.0 W/(m2-K). The outside surface temperature of the door is 58.0°C. Find the heat transfer rate q, in units of Watts, associated with this cooling process.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY

Elements Of Electromagnetics
Mechanical Engineering
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:9780134319650
Author:Russell C. Hibbeler
Publisher:PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:9781259822674
Author:Yunus A. Cengel Dr., Michael A. Boles
Publisher:McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:9781118170519
Author:Norman S. Nise
Publisher:WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:9781337093347
Author:Barry J. Goodno, James M. Gere
Publisher:Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:9781118807330
Author:James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:WILEY