
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
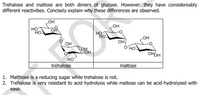
Transcribed Image Text:Trehalose and maltose are both dimers of glucose. However, they have considereably
different reactivities. Concisely explain why these differences are observed.
OH
но
но
но
OH
LOH
он
O'HO-
он
OH.
OHOH
HO
trehalose
maltose
1. Malthose is a reducing sugar while trehalose is not.
2. Trehalose is very resistant to acid hydrolysis while maltose can be acid-hydrolyzed with
ease.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Lactose is a disaccharide in which a glycosidic linkage connects the monosaccharides galactose and glucose. OH НО OH (a) Identify the glycosidic linkage and the acetal carbon in lactose. (b) What type of glycosidic linkage does lactose have (i.e., is it 1,1'-, 1,2'-, etc., and is it a or B)? (c) People who are lactose intolerant are deficient in the enzyme lactase, and therefore cannot efficiently break down the disaccharide into its monosaccharides. When lactose is treated with aqueous acid, however, this hydrolysis can take place, though relatively slowly. Draw the complete, detailed mechanism and the products of the acid-catalyzed hydrolysis of lactose. Но ОН НО ОН ОН Lactosearrow_forward1. Consider the following molecules. Clearly circle the glycosidic bond on each structure. In the Spae provided clearly describe the type of glycosidic bond illustrated. HO-CH2 O. H. H. OH HO. HO-CH2 OH H. H. H. OH H. OH Но H. H. H. OH OH HOH2Ç HO OH OH CH2OH HI エー エエーarrow_forwardIn solution, glucose is mostly found in the cyclic hemiacetal state, which lacks an aldehyde group. How are mild oxidising agents able to oxidise glucose?arrow_forward
- Consider N-acetyl-d-glucosamine Q.) Draw a chair conformation for the disaccharide formed by joining two units of the pyranose form of N-acetyl-d-glucosamine by a b-1,4-glycosidic bond. If you draw this correctly, you have the structural formula for the repeating dimer of chitin, the structural polysaccharide component of the shell of lobsters and other crustaceans.arrow_forwardUsing the attached image answer the following questions Which one of A,B,C & D is frutcopyranose? Which structure represents a ketose?arrow_forwardThe following trisaccharide derivative is important to human health.The A ring is a deoxy derivative of which aldohexose?arrow_forward
- 2. Draw the two aldohexoses and one ketohexose that can be derived from the enol shown below. HC OH но H- HO- HO ČH,OH 3. Classify the following monosaccharides as reducing and nonreducing sugars. CH,OH CH,OH CH,OH OH он но - HO- OH OH HO он OH OH ČH,OH II III 4. Identify and name the glycosidic bond in the structure shown below. CH,OH OH OH онarrow_forwardI need help on number 6, a-farrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY