
College Physics
11th Edition
ISBN: 9781305952300
Author: Raymond A. Serway, Chris Vuille
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
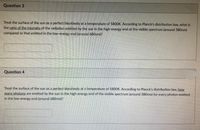
Transcribed Image Text:Question 3
Treat the surface of the sun as a perfect blackbody at a temperature of 5800K. According to Planck's distribution law, what is
the ratio of the intensity of the radiation emitted by the sun in the high-energy end of the visible spectrum (around 380nm)
compared to that emitted in the low-energy end (around 680nm)?
Question 4
Treat the surface of the sun as a perfect blackbody at a temperature of 580OK. According to Planck's distribution law. how
many photons are emitted by the sun in the high-energy end of the visible spectrum (around 380nm) for every photon emitted
in the low-energy end (around 680nm)?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- The blackbody radiation emitted from a furnace peaks at the wavelenght of 1.2 x 10^-6 m (0.0000012 m). What is the temperature inside the furnace?arrow_forwardPlanck’s constant has the value h = 6.626 × 10–34 joule-seconds (J-s), and the speed of light is c = 3 × 108 m/s. Using these values, calculate the wavelength carried by photons emitted with an energy of 1.1 × 10-19 J.arrow_forwardAs noted in the chapter, the cosmic microwave background radiation fits the Planck equation for a blackbody at 2.7 K. (a) What is the wavelength at the maximum intensity of the spectrum of the background radiation? (b) What is the frequency of the radiation at the maximum? (c) What is the total power incident on Earth from the background radiationarrow_forward
- For light with a wavelength of 350 nm and with an intensity of /= 10-8 W/m², what is the number of photons/(m²s) in the light beam?arrow_forwardA blackbody is brightest at a wavelength of 800 nm. At what wavelength would the blackbody be brightest if its temperature were tripled?arrow_forwardWhat is the wavelength of a photon emitted when an electron jumps from the n=3 to the n=2 energy levels of a lithium atom (Z=3)? Express your answer in nanometers and keep three significant digits.arrow_forward
- Calculate the energy of a photon of frequency 3.5 x 1015 Hz, in electronvolts (eV). Do an online search to find the conversion factor between electronvolts and joules.arrow_forwardUnexcited hydrogen atoms are bombarded with electrons that have been accelerated through 12.5 V. What is the highest energy level that the hydrogen atoms will be excited to? When the electron of the hydrogen atom is excited into the level in part a), what wavelength(s) is (are) possible for the photon emitted when the electron drops to a lower energy level? [Answer in 3 significant figures] a) b)arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON
University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press
Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley
Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON
College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON

College Physics
Physics
ISBN:9781305952300
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning

University Physics (14th Edition)
Physics
ISBN:9780133969290
Author:Hugh D. Young, Roger A. Freedman
Publisher:PEARSON

Introduction To Quantum Mechanics
Physics
ISBN:9781107189638
Author:Griffiths, David J., Schroeter, Darrell F.
Publisher:Cambridge University Press

Physics for Scientists and Engineers
Physics
ISBN:9781337553278
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Lecture- Tutorials for Introductory Astronomy
Physics
ISBN:9780321820464
Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina Brissenden
Publisher:Addison-Wesley

College Physics: A Strategic Approach (4th Editio...
Physics
ISBN:9780134609034
Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart Field
Publisher:PEARSON