
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Figures 2 to 4 show the IR, 1H NMR and 13C NMR spectra of a compound with formula C3H5N
2a) Identify bands A and B in the IR spectrum
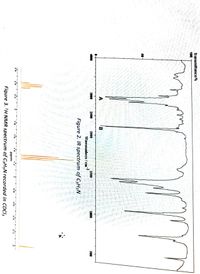
Transcribed Image Text:Transmittance/%
100
50
A
4000
3000
2500
2000
1500
1800
500
Wavenumbers / cm
Figure 2. IR spectrum of C3H5N
26
2'4
1'8
1'6
1'4
1'2
22
o's
o'2
ppm
Figure 3. H NMR spectrum of C3H5N recorded in CDCI3

Transcribed Image Text:122
121
120
119
118
117
116
12
10
ppm
Figure 4. 13C NMR spectrum of C3H,N in CDCI3. The figure shows only the sections in which peaks appear.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Mass spectrometry of an unknown compound revealed a molecular ion at m/z 100.09. The IR spectrum, the ¹³C NMR spectrum, and the ¹H NMR spectrum are shown below. Draw the structure of this compound. 4000 220 3000 200 180 160 2000 M 1500 Wavenumbers (cm-¹) 140 120 100 1H 80 6H 1000 40 3H 20 ppm 500arrow_forwardUse the 1H NMR and IR spectra given below to identify the structure of compound B (molecular formula C4H8O2).arrow_forwardWhat best describes the IR spectrum of NaCl? A broad peak at 3400 cm-1 A strong peak at 1700 cm-1 No peaks are observed A weak peak just above 3000 cm-1arrow_forward
- A student prepares a compound that she believes has the molecular formula C4H1002. Give a possible structure for this compound using the information from the following spectra. How many peaks will your proposed structure give in the 13C NMR? 1Η NMR singlet 3.25 (3 H) singlet 3.45 (2 H) IR nothing at 3500 cm-1 nothing at 1700 cm-1arrow_forwardThe NMR spectra for compound 1 were acquired in a 7.5 mg / 0.6 mL solution ofCDCl3 . The 1H and 13C peaks are also listedbelow. Provide a full analysis of the NMR spectra for compound 1. correct assignment of NMR spectra of both 13C spectra. correct rationalisation of 13C spectrum1H NMR (400 MHz, CDCl3) δ 7.73 (d, J = 9.5 Hz, 1H), 7.56 (ddd, J = 8.5, 7.5, 1.6 Hz, 1H),7.51 (dd, J = 7.5, 1.6 Hz, 1H), 7.36 (d, J = 8.5 Hz, 1H), 7.30 (dd, J = 8.5, 7.5 Hz, 1H), 6.45(d, J = 9.5 Hz, 1H).13C NMR (101 MHz, CDCl3) δ 160.79, 154.09, 143.43, 131.85, 127.87, 124.44, 118.86,116.94, 116.74.Note: There are two carbon peaks in the 13C spectrum that are so close together that they are not differentiable at the resolution in this experiment. you should be able to assign these peaks to one of two carbon atoms in 1.arrow_forwardFor the protons labeled Hª and Hb in the structure, predict the characteristics of their signals in the ¹H NMR spectrum: the approximate chemical shift, the splitting pattern, and the integration value. Ha a TOT a нь Нь Xarrow_forward
- The NMR spectra for compound 1 were acquired in a 7.5 mg / 0.6 mL solution ofCDCl3 and are found in the accompanying file. The 1H are also listedbelow. Identify the name of compound 1 and Provide a full analysis of the NMR spectra for compound 1 with table showing peak name and assignment 1H NMR (400 MHz, CDCl3) δ 7.73 (d, J = 9.5 Hz, 1H), 7.56 (ddd, J = 8.5, 7.5, 1.6 Hz, 1H),7.51 (dd, J = 7.5, 1.6 Hz, 1H), 7.36 (d, J = 8.5 Hz, 1H), 7.30 (dd, J = 8.5, 7.5 Hz, 1H), 6.45(d, J = 9.5 Hz, 1H).arrow_forwardElemental analysis determined unknown Compound G to contain 40.0 % carbon, 6.7 % hydrogen. Compound G had an M+ = 60 m/z on its MS. Its IR displayed three absorbances: strong at 1710, strong below 3000, and broad from 2500 to 3500. The proton NMR showed an absorbance at 1.7 ppm (lowest whole number ratio of H =3), and an absorbance at 11.5 ppm (lowest whole number ratio of H = 1). Compound G was able to turn blue litmus paper red, and neutralize a base. What is Compound G? CH3CO2H Formaldehyde HO \ C = CH2 / HO CH2(OH)CHO CH(OH)=CH(OH)arrow_forwardA compound displays a sharp doublet at 3400 cm-1 in its IR spectrum. The mass spectrum has a molecular ion with m/z = 115 and a base peak of m/z=72. Draw a structure that best fits this data.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY