
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
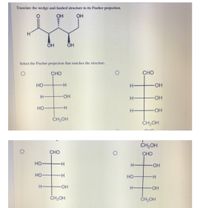
Transcribed Image Text:Translate the wedge-and-hashed structure to its Fischer projection.
OH
OH
H.
OH
Select the Fischer projection that matches the structure.
CHO
CHO
Но-
H-
-HO-
H-
HO-
HO
но
H-
HO
ČH2OH
CH,OH
CH,OH
CHO
CHO
но-
H-
HO.
HO
Но
HO-
CH2OH
CH2OH
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Draw the Fischer projection from the chair conformation shown below.arrow_forwardDraw the final product and identify R or S or racemic in all chiral centers in the product. Ph CH3 (R) + NaN3 acetone solvent H3C (S) Draw the final product and identify R or S or racemic in all chiral centers in the product. Ph CH3 (R) O. O NAOCH3 THF solvent H3C (S)arrow_forwardTranslate the Fischer projection to a wedge-and-hashed bond structure in a zig-zag chain. Be sure each stereocenter clearly shows one wedged bond, one hashed bond and two in-plane bonds. Select Draw Rings More Erase H |||| с Q2 Q H HO HO HO -OH -H -H -H CH₂OH H Oarrow_forward
- Identify the C3 epimer of the sugar below drawn in its open chain (acyclic) Fischer projection. OH OH тон OH OH A) CHO H-OH H-OH НО -H Н- B) CHO HO-H H-OH -OH CH₂OH HO- -Н H-OH C) Н НО НО сн он CHO HO- OH -Н -H H-OH он он D) HO -H HO -H CHO H-OH -H CH₂OHarrow_forwardWhich fischer projection represents the following haworth projection? H₂C-OH CH-O HO OH C HOOH HAH C-C H OH о H₂C LOH C=0 но-с-он H-C-OH H-C-OH H₂C OH H HO HO H- HO -OH H HO HO 애애 О LOH H₂C C=0 H-C-OH HO-C-OH H-C-OH H₂C. HO HO -OH 11 ITarrow_forwardНО Но DA Which Fischer diagram represents the molecule shown below? с в D E Н -CH3 -Et CH3 CH3 -ОН Et- -ОН т H3C в Н. H3C о Et НО ОН ОН Н Et CH3 C CH3 -CH3 ОН Но но- CH3 Н -СН3 -Et Н но- -CH3 # Et- ОН CH3 Earrow_forward
- Which of these Fischer projections is correctly represents the given Haworth projection:arrow_forwardCheck the box under each L-ketose. If there are none, check the None of the above box under the table. H H H OH Н OH CH₂OH П U Н. H НО- H H -OH H OH -OH CH₂OH НО H CH₂OH c=0 OH CH₂OH U H H H НО 애애애 H CH₂OH U НО НО CH₂OH c=0 H -Н CH₂OHarrow_forwardConvert product C, shown below, into its Fischer projection. One of the carbon atoms has been marked with a star (*) to allow you to keep track of it. Which of the atoms numbered 21-24 are bromine? H Br D C 21 22 23 24 22 000arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY