
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
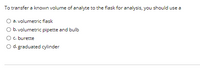
Transcribed Image Text:To transfer a known volume of analyte to the flask for analysis, you should use a
a. volumetric flask
O b. volumetric pipette and bulb
Oc. burette
O d. graduated cylinder
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What volume of 0.1524 M NaOH is required to neutralize 50.00 mL of 0.1016 M HClarrow_forwardame: Table 2. Determination of Acetylsalicylic Acid in Aspirin. 1) Aspirin Brand (and amount of acetylsalicylic acid on label) 2) Average molar concentration of NaOH Solution B (from Part A) 3) 4) 5) 6) Mass of aspirin tablet Mass of pulverized aspirin sample Initial buret reading Final buret reading 7) Volume of NaOH Solution B added 8) Moles of NaOH 9) Moles of acetylsalicylic acid in sample 10) Mass of acetylsalicylic acid in sample 11) Amount of acetylsalicylic acid per tablet T₁ 1.264 12) Mean amount of acetylsalicylic acid per tablet 13) Standard deviation for acetylsalicylic acid per tablet 14) RSD for acetylsalicylic acid per tablet 15) Relative percent error (%) 16) Is the mean amount per tablet acceptable? (Yes or no.) 0.000153 * 180. Kg Imol T₂ √3 1.261 1-259 Seat กา Basic 8.25 mg 0.0975 M Trial 1* Trial 2 *Show all calculations related to Trial 1, standard deviation, RSD, and relative % error. Mole of NaOH (0.0975) (14.3) = 0.000139 (0.0975) (15.7) = 8.000 253 23 0.0975)…arrow_forwardPlease provide steps so I understand how to do pleasearrow_forward
- You have a 2.00 M solution of sucrose and need to use it to make 1.0 L of a 0.500 M solution of sucrose. You will take _____ mL of that 2.00 M solution and add enough water to make 1.00 L of the new solution.arrow_forwardA laboratory stock solution has a molar concentration of 6.70 M NaOH. Calculate the volume (in milliliter) of this stocksolution that would be needed to prepare 583 milliliters of 0.395 M NaOH. A. 34.4 B. 26.9 C.41.9 D.19.4 E.49.4arrow_forward101) X -p.101edu.co - Hiring Ce... F4 144 $ 4 Aktiv Chemistry F5 PU 25 F6 A k How many moles of sodium chloride are there in 0.55 L of a 1.60 M aqueous solution of sodium chloride? A 6 X A) 2.90 Login - Labster B) 0.344 C) 0.880 F7 D) 4.20 F8 7 X Question 9 of 9 F9 88 11. Erikson's Psychosocial Theory o X DELL F10 9 F11 8 O F12 + PrtScr T DE Insert Dele Backarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY