
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
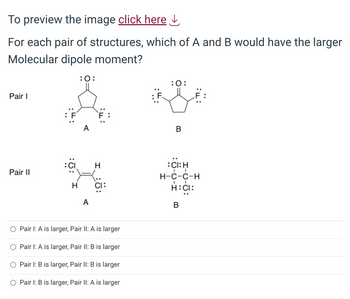
Transcribed Image Text:To preview the image click here ↓
For each pair of structures, which of A and B would have the larger
Molecular dipole moment?
Pair I
Pair II
:CI
H
:0:
A
H
CI:
Pair I: A is larger, Pair II: A is larger
Pair I: A is larger, Pair II: B is larger
Pair I: B is larger, Pair II: B is larger
Pair 1: B is larger, Pair II: A is larger
:0:
B
:CI: H
I
H-C-C-H
I
H:CI:
B
..
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Question 4 of 18 Macmillan Learning > Essentials of General, Organic, and Biochemistry Denise Guinn THIRD EDITION Isoflurane is used as an inhaled anesthetic. The image shows the Lewis dot structure of isoflurane. F: :CI: :F: 2 :F: Q. :F: H 120° 109.5° 180° 90° C :O: O linear ·C H What is the bond angle around each carbon center? :ד: What is the molecular geometry at each carbon center? What is the bond angle around the oxygen center? 180° 90° O 120° 109.5° presented by Macmillan Learning What is the molecular geometry at the oxygen center? O trigonal planararrow_forwardRank the following structures I, II, III in order of increasing energy of the nonbonding electrons. H B (N-H o-bond) A (C=C π-bond) Aarrow_forwardWhich of the following bonds is least polar? OC-O OH-C S-CI r-Br sy are all nonpolar. 17arrow_forward
- H3C N(CH3)2 Draw the molecule on the canvas by choosing buttons from the Tools (for bonds), Atoms, and Advanced Temp toolbars. The single bond is active by default. DDC H2D EXP. CONT. 3 [1] A H₂C NH™ H C N O S CI Br I P F LLarrow_forwardPlease don't provide hadnwritten solutionarrow_forward10:27 : Br - Br: HIN: H-N-H a-F: 4G .ill 60% The highlighted bond is polar and the more negative atom is The highlighted bond is nonpolar The highlighted bond is polar and the more negative atom is O The highlighted bond is nonpolar. X O The highlighted bond is polar and the more negative atom is The highlighted bond is nonpolar.arrow_forward
- 11:54 9 Question 48 of 53 Submit Draw the Lewis structure for thiosulfate (S203) with minimized formal changes. How many TOTAL likely resonance structures exist for S2032-? Hint: In this case, it is more stable (preferred) to place a negative charge on the larger atom. + Click to draw a new structure A) 1arrow_forwardPLS HELP ASAParrow_forward16arrow_forward
- 14 ✪ ✪ Dec 28 9:29 O Dot formula is an exception to Octet rules. Formula Dot Requirement Sef4 Dot Formula N Geometry 8D Dondo (4a) Bonds ! showbond angles Molecular Geometry (7a) (76) (7b) 7b) $1 z < 2023-12-19_11-15.pdf 12 / 14 TT xli T Ć :arrow_forwardPls helo ASAP. Pls help in all asked questions.arrow_forwardHow much energy (in kJ) is required to separate (break) one mole of H-H bonds?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY