
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
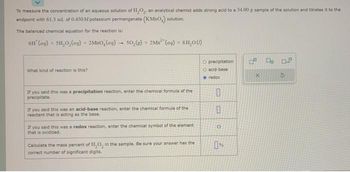
Transcribed Image Text:To measure the concentration of an aqueous solution of H₂O₂, an analytical chemist adds strong acid to a 34.00 g sample of the solution and titrates it to the
andpoint with 61.3 ml. of 0.450M potassium permanganat (KMnO,) solution.
The balanced chemical equation for the reaction is:
6H (aq) + SH₂O₂(aq)
+
What kind of reaction is this?
2MBO (ag) 50,(g) + 2Mn (aq) +8H₂0 (1)
-
If you said this was a precipitation reaction, enter the chemical formula of the
precipitate.
If you said this was an acid-base reaction, enter the chemical formula of the
reactant that is acting as the base.
If you said this was a redox reaction, enter the chemical symbol of the element
that is oxidized.
Calculate the mass percent of H₂O, in the sample. Be sure your answer has the
correct number of significant digits.
O precipitation
O acid-base
redox
0
0
0%
X
Co 0.2
S
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 6 steps with 36 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A sample of iron ore is dissolved in acid, and the iron is converted to Fe2*. The sample is then titrated with 47.20 mL of 0.02240 M MnO4 solution. The oxidation-reduction reaction that occurs during titration is as follows: MnO4(aq) + 5FE2*(aq) + 8H*(aq) → Mn²*(aq) + 5FE3* (aq) + 4H2O How many moles of MnO4¯ were added to the solution? Blank 1 2+ How many moles of Fe were in the sample? Blank 2 How many grams of iron were in the sample? Blank 3 If the sample had a mass of 0.8890 g, what is the percentage of iron in the sample? Blank 4 Blank 1 Add your answer Blank 2 Add your answer Blank 3 Add your answer Blank 4 Add your answerarrow_forwardThe quantity of antimony in a sample can be determined by an oxidation–reduction titration with an oxidizing agent. A 6.19 g6.19 g sample of stibnite, an ore of antimony, is dissolved in hot, concentrated HCl(aq) and passed over a reducing agent so that all the antimony is in the form Sb3+(aq). The Sb3+(aq) is completely oxidized by 30.5 mL of a 0.115 M aqueous solution of KBrO3(aq). The unbalanced equation for the reaction is BrO^− 3(aq) + Sb^3+(aq)⟶Br^−(aq)+Sb^5+(aq)(unbalanced) Calculate the amount of antimony in the sample and its percentage in the ore. mass of antimony in grams: percentage of antimony:arrow_forwardThe following chemical reaction takes place in aqueous solution: SnBr₂(aq) +2 KOH(aq) → Sn(OH)₂(s)+2 KBr(aq) Write the net ionic equation for this reaction.arrow_forward
- Consider the neutralization reaction 2HNO3(aq) + Ba(OH)2(aq) yields to 2H2O(L) + Ba(NO3)2(aq) A 0.100 L sample of an unknow HNO3 solution required 49.3 mL of 0.100 M Ba(OH)2 for complete neutralization. What is the concentration of the HNO3 solution?arrow_forwardA sample of a chloride salt weighing 484.6 mg is titrated with AgNO3 using K2CrO4 as indicator. To reach the end point a 52.3 mL of 0.1475 M of AgNO3 solution is required. The net ionic equation for the reaction of titration is: Ag+ (aq) + Cℓ- (aq) → AgCℓ (s) How many moles of Ag+ (aq) ions are used to reach the end point? How many moles of Cℓ- (aq) have reacted in the titration? How many grams of chloride are present in the sample? What is the mass percent of chloride in the sample?arrow_forwardThe zinc content of a 1.55 g ore sample was determined by dissolving the ore in HCl, which reacts with the zinc. The excess HCl is then neutralized with with NaOH. The reaction of HClHCl with Zn is shown. Zn(s)+2HCl(aq)⟶ZnCl2(aq)+H2(g) The ore was dissolved in 150 mL of 0.600 M HCl and the resulting solution was diluted to a total volume of 300 mL. A 20.0 mL aliquot of the final solution required 8.92 mL of 0.548 NaOH to neutralize the excess HCl. What is the mass percentage (%w/w) of Zn in the ore sample?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY