Concept explainers
Question
Transcribed Image Text:eq
eq
req
req
2req
M
s2req
ts 2req
✓
O
3
F3
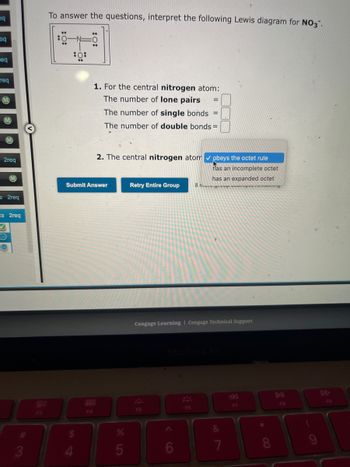
To answer the questions, interpret the following Lewis diagram for NO3".
*
:0:
**
:0
1. For the central nitrogen atom:
The number of lone pairs =
The number of single bonds =
The number of double bonds=
F4
2. The central nitrogen atom ✔ obeys the octet rule
has an incomplete octet
has an expanded octet
manny
Submit Answer
%
5
Retry Entire Group 8
Cengage Learning Cengage Technical Support
F5
F6
&
7
F7
DII
FB
F9
Transcribed Image Text:=q
req
Preq
Visited
F3
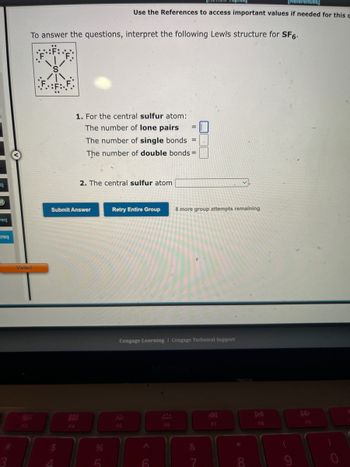
To answer the questions, interpret the following Lewis structure for SF6.
F4
1. For the central sulfur atom:
The number of lone pairs
The number of single bonds =
The number of double bonds=
Submit Answer
[References)
Use the References to access important values if needed for this a
2. The central sulfur atom
%
Retry Entire Group 8 more group attempts remaining
Cengage Learning Cengage Technical Support
F5
A
F6
&
F7
*
∞
F8
F9
Expert Solution
arrow_forward
Step 1
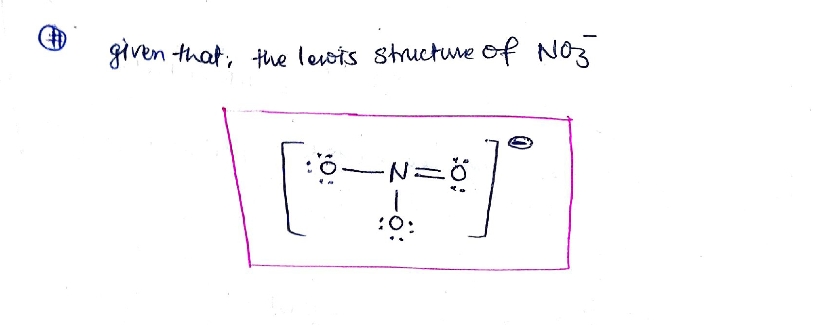
Answer :
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.