
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Please correct answer and don't use hand rating
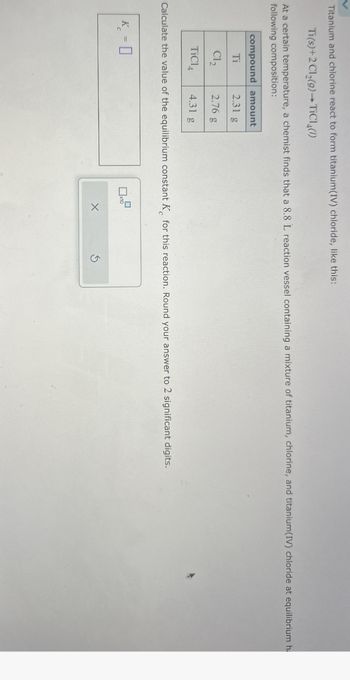
Transcribed Image Text:Titanium and chlorine react to form titanium(IV) chloride, like this:
Ti(s)+2Cl2(9) TiCl4(1)
At a certain temperature, a chemist finds that a 8.8 L reaction vessel containing a mixture of titanium, chlorine, and titanium(IV) chloride at equilibrium h
following composition:
compound amount
Ti
2.31 g
Cl₂
2.76 g
TiCl
4.31 g
Calculate the value of the equilibrium constant K for this reaction. Round your answer to 2 significant digits.
K-0
x10
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- Part C Li + then H₂O+ quencharrow_forwardQuality control material in the hematology laboratory is best described as: Question 3 options: A) a stable material used to establish the accuracy of an automated method and normal curve B) a stable material used to monitor the functioning of an analyzer or the performance of a process C) reagents used to perform preventative maintenance D) materials tested to develop a normal reference rangearrow_forwardINSTRUCTIONS 1. Please enumerate all given values. 2. Identify all unknown values 3. Write the formula first before substituting the values. 4. Enclose final answers in boxes. 5. Do not round off immediately. Consider the whole value during computations. 6. Round off final answers to 2 DECIMAL PLACES ONLY. Problem: What volume (mL) sulfuric acid solution (98.0% of the concentrated sulfuric acid by weight; density 1.84 g/mL) and the volume of water are used in the preparation of 2.00 L of a 4.00 N solution?arrow_forward
- The solvent front distance is from ... Group of answer choices the bottom of the paper to the top of where the solvent reached. the baseline to the top of where the solvent reached. from the top of the spot to the top of the paper. the bottom of the paper to the top of the paper. from the top of the spot to the top of where the solvent reached.arrow_forwardDraw the sktech graph i want to know if i did it rightarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY