
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
Give a clear handwritten answer...please give answer all sub parts?
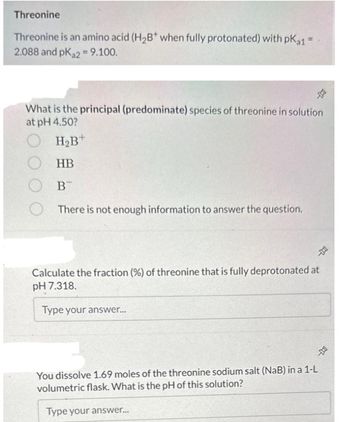
Transcribed Image Text:Threonine
Threonine is an amino acid (H₂B* when fully protonated) with pKa1 =
2.088 and pKa2 = 9.100.
D
What is the principal (predominate) species of threonine in solution
at pH 4.50?
OH₂B+
OHB
B
There is not enough information to answer the question.
-
Calculate the fraction (%) of threonine that is fully deprotonated at
pH 7.318.
Type your answer...
DO
You dissolve 1.69 moles of the threonine sodium salt (NaB) in a 1-L
volumetric flask. What is the pH of this solution?
Type your answer...
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- One disadvantage of thin layer chromatography is that... it cannot be used for quantitative measurements. O it consumes large volumes of solvents O it cannot analyze volatile samples it cannot be used to analyze many samples simultaneously it requires a large amount of sample for analysisarrow_forwardCompare Chromatographic and Spectroscopic methods for pharmaceutical analysis? Please answer at your own easy words. Answer should be to the point.arrow_forwardThe purpose of adding sodium sulfate in the extraction is to decrease the solubility of caffeine in dichloromethane. Hence, the residue could contain water and other impurities. a First statement is true. Second statement is false. b First statement is true. Second statement is false. c Both statements are false. d Both statements are true.arrow_forward
- A www-awu.aleks.com/alekscgi/x/lsl.exe/1o_u-IgNslkr7j8P3jH-IJgXZp57itWHhRgilODc5Mqv... G Apps CSU Study/Teach Abro... GACE Testing O Degree Related E E-Tandem E Grade Calculator 国Re Korean O STATES OF MATTER Identifying the intermolecular forces between atoms, ions and... Kyli. What kind of intermolecular forces act between a bromine (Br,) molecule and a tetrachloroethylene (C,Cl4) molecule? Note: If there is more than one type of intermolecular force that acts, be sure to list them all, with a comma between the name of each force.arrow_forwardGive detailed Solution..show work..don't give Handwritten answer..don't use Ai for answering this..don't copy answer anywherearrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY