
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
explain the answer using the hess's law (gr 13) please type if possible
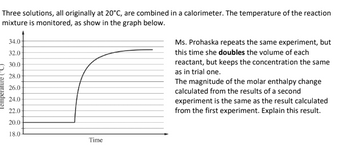
Transcribed Image Text:Three solutions, all originally at 20°C, are combined in a calorimeter. The temperature of the reaction
mixture is monitored, as show in the graph below.
Temperature (°C)
34.0-
32.0-
30.0-
28.0-
26.0-
24.0-
22.0-
20.0-
18.0
Time
Ms. Prohaska repeats the same experiment, but
this time she doubles the volume of each
reactant, but keeps the concentration the same
as in trial one.
The magnitude of the molar enthalpy change
calculated from the results of a second
experiment is the same as the result calculated
from the first experiment. Explain this result.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 4 steps with 6 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The temperature of a gas is a measure of the average kinetic energy of the gas molecules O True Falsearrow_forward53. Consider the initial. and final data for an ideal gas: Which expression gives the final volume, V2, in liters? Initial Final 3 atm 2L Temperature 300 K Pressure Volume 5 atm ?L 400 K (A) 2x 중x0 400 300 (B) 2× x 400 300 400 300 (D) 2x 를x200arrow_forward18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 If the temperature is doubled from 20.0°C and the original pressure is 732 Torr, what will the new pressure be? Show all of your work and include the correct units. * Student can enter max 3000 characters XD BI U = |Q| Use the paperclip button below to attach files. M 36 of 41 G Sign outarrow_forward
- Use the ideal gas law to verify the triple product rule.arrow_forwardplease help me, double check your answers previous tutors got it wrong.arrow_forwardHow hot must you heat 369 mL of gas at 33.3°C for it to expand to 3.6 L? Express your answer in Kelvin and round to 1 decimal place. Be sure to use the proper abbreviation of the UNITSarrow_forward
- Write the acidic equilibrium equation for CSHSNH.arrow_forward&drc-D0&gi-24758088- A flexible lighting fixture is filled with neon. The starting volume of the fixture is 8.2L and the pressure of the neon is 3.2 atm. If the fixture is compressed, reducing the volume to 1.6L, what is the new pressure in the lamp? Your Answer: Answer units D Add attachments to support your workarrow_forwardA sample of gas is at a pressure of 2.81 atm, 664K and a volume of 713L. What will be the pressure of the gas if it's volume and temperature change to 126L and 368K respectively? (show work)arrow_forward
- According to KMT, the measure of gas particles' kinetic energy is related to_____arrow_forwardneed help with this question please teacher didn’t explain wellarrow_forwardIn this experiment, we will be using the gas inside of a small hand-held lighter. Before the trial, the lighter weighed 46.747 g. After the trial the mass of the lebter was 31.104 g. what is the mass in grams of the gas used? Enter a numeric answer only. A Moving to another question will save this response. «< Question 6 of 10 8:39 AM search 耳 a 22 4/13/2022 hp insert prt sc delete 144 %24 4 %23 & L. 8 6. %3D backspace hor R T Y U P F G L enter D pause t sh C alt ctriarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY