
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
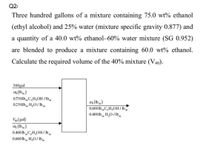
Transcribed Image Text:Q2/
Three hundred gallons of a mixture containing 75.0 wt% ethanol
(ethyl alcohol) and 25% water (mixture specific gravity 0.877) and
a quantity of a 40.0 wt% ethanol–60% water mixture (SG 0.952)
are blended to produce a mixture containing 60.0 wt% ethanol.
Calculate the required volume of the 40% mixture (V40).
300gal
m (lb)
0.750lbC,H,OH /lb
m, (Ib)
0.600 lb„C,H,OH/ Ib.
0.400 lb H,0/lbm
0250lb, H,0/ lb.
Vao (gal)
m (lb)
0.400 lb „C,H,OH/lb,
0.600 lb H,O/lb
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemical-engineering and related others by exploring similar questions and additional content below.Similar questions
- Calculate density in the following cases : (a) Air (ideal gas) at temperature 0°C and pressure 2 atm (b) Helium (ideal gas) that fills a 10-m³ volume at temperature 25°C, whose gauge pressure reads 1.5 atm (c) Granular iron of spherical grains of average diameter D = 1mm packed in a 1-grain/mm3 arrangement (density of iron p = 9g/cm³). Assume that the void in each mm3 is filled with air. Discuss why the result is expected. Make the same computation if the average diameter of the spherical grains are D = 0.25 mm packed in a 64-grain/mm3 . Discuss why the result for the density of both arrangements is the same.arrow_forwardCalculate the freezing point of a 2.0m solution of NaCl in water? Remember that NaCl is an electrolyte. The normal freezing point of pure water is 0.00°C. Kf(water) =1.86°C/marrow_forwardCalculate the number of moles and the mass of the solute in 10.5 L of 3.716 M (NH4)2SO4, a liquid fertilizer.arrow_forward
- Using the balanced chemical equation 2C5H8N4O12 + 2O2 -> 4N2 +8H2O + 10 CO2 calculate the volume (in liters) of gas that your explosive would produce if 1 g exploded and resulted in the products shown in your chemical equation. Assume standard temperature and pressure (STP) and any water produced is in the gaseous state.arrow_forward3.43.0 g of 40 Ar gas are sealed in a container at an initial pressure of 1.50 atm and an initial volume of 0.0500 m³ (state 1). The gas is then made to expand very, very quickly until its volume doubles (state 2). Then it is compressed very, very slowly back to its initial volume (state 3). Show the two processes on a pV diagram, and fill out the table below. Be sure to show your work for full credit. P1 = V₁ = T₁ = P2 = V₂ = T₂ = P3 = V3 = T3 =arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The