
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Transcribed Image Text:T
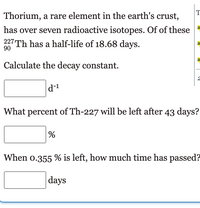
Thorium, a rare element in the earth's crust,
has over seven radioactive isotopes. Of of these
227 Th has a half-life of 18.68 days.
90
Calculate the decay constant.
What percent of Th-227 will be left after 43 days?
When o.355 % is left, how much time has passed?
|days
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 25°PU is a nuclear waste byproduct with a half-life of 24,000 y. What fraction of "Pu present today will be present in 1000 y? 88% B .973% 97.3% D .888%arrow_forwardCobalt-60 is a radioactive isotope that is widely utilized in nuclear medicine as a -ray source. It is formed through a neutron capture reaction with cobalt-59 and is a ß emitter; ß emission is associated with intense radiation. If Co-60 has a half-life of 5.27 years: a. How long will it take for the Co-60 source to be reduced to one-eighth of its initial concentration? b. What percentage of the Co-60 source will remain after three years (rounded to two decimal places)?arrow_forwardQuestion 18: Uranium-232 is a radioactive isotope with a half life of 68.9 years. How long in years will it take for 40% of a sample of U-232 to decay? 51.1 x yearsarrow_forward
- If 25%(1/4)of a radioactive element remains after1,000years, what is the half-life?arrow_forwardIodine-131 is a radionuclide that is frequently used in nuclear medicine. Among other things, it is used to detect fluid build up in the brain. The half-life of iodeine-131 is 8.0 days. How much of a 0.16-g sample of iodine-131 will remain undecayed after a period of 32 days? a.) 0.020g b.) 0.080g c.) 0.010g d.) 0.040garrow_forwardThe radioactive isotope I-131is used in the treatment of hyperthyroidism. When administeredto a patient, I-131 accumulates in the thyroid gland, where it decays and kills part of thatgland. Consider the half-life of 8 days.a) Suppose that it takes 72 hours to ship I-131 from the producer to the hospital. Whatpercentage of the original amount shipped actually arrives at the hospital.b) If the I-131 is stored at the hospital for an addition 48 hours before it is used, how muchof the original amount shipped from the producer is left when it is used?c) How long will it take for the I-131 to decay completely so that the remnants can be thrownaway without special precautions?arrow_forward
- What is the half‑life of an isotope that decays to 3.125%3.125% of its original activity in 41.8 h?arrow_forwardHow many hours are needed for a 16.0 g sample of Si-31 to decay to 2.0 g? The half-life for Si-31 is 2.6 h.arrow_forwardRadioactive vanadium-48 decays with a half-life of 16.0 days. a. What is the value of k in s? k = b. A sample contains 84.0 mg *°V. How many decay events will be produced in the first second? Assume the atomic mass of **V is 48.0 u. decays/s c. A chemist obtains a fresh sample of 48 V and measures its radioactivity. She then determines that to do an experiment, the radioactivity cannot fall below 25% of the initial measured value. How long does she have to do the experiment? daysarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY