
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
This question already has all soltions. Please show the steps how the answers m% in red was calculated, m%=mass %
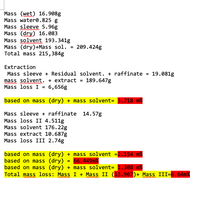
Transcribed Image Text:Mass (wet) 16.908g
Mass watere.825 g
Mass sleeve 5.96g
Mass (dry) 16.083
Mass solvent 193.341g
Mass (dry)+Mass sol. = 209.424g
Total mass 215,384g
Extraction
Mass sleeve + Residual solvent. + raffinate = 19.081g
mass solvent. + extract = 189.647g
Mass loss I = 6,656g
based on mass (dry) + mass solvent= 3.718 m%
Mass sleeve + raffinate 14.57g
Mass loss II 4.511g
Mass solvent 176.22g
Mass extract 10.687g
Mass loss III 2.74g
based on mass (dry) + mass solvent =2.154 m%
based on mass (dry)
based on mass (dry) + mass solvent= 1. 308 m%
Total mass loss: Mass I + Mass II (13.907)+ Mass III=6.64m%
66.449m%
%3D
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Determine the percentage by mass of each element in the following compounds. (Round your answers to one decimal place.) (a) water, H2O X % X % (b) washing soda, Na,CO3 Na X % C X % X %arrow_forwardShow step by step solution/explanation PERCENT COMPOSITIONFind the percent composition of all the elements in the following compounds;1. Mg(NO3)2 2. CuBr EMPIRICAL FORMULA1. Find the empirical formula for a compound which contains 32.8% chromium and 67.2% chlorine. MOLECULAR FORMULA1. The empirical formula of a compound phosphorous and oxygen was found to be P2O5.The molar mass of this compound is 283.88 g/mol. What is the compound’s molecular formula?arrow_forwardHydrogen peroxide , H_{2}*O_{2} is a colorless liquid . A concentrated solution of it is used as a source of oxygen for rocket propellant fuels . If the solution has 0.901 mols of H_{2}*O_{2} what is the mass of H 2 O 2 ? Answer to decimal places and include the unit .arrow_forward
- bctions: Show your solution in the space provided. Follow the GFFSA (Given, Find, ymula, Solution, Answer) format if possible. Write your answers in the space provided. 1. Combustion of hydrocarbons like methane (CH4) produces two products, water (H2O) and carbon dioxide (CO2). Consider the chemical equation of this combustion: CHA + 2 02 - CO2 + 2 H2O, when 4 mol of CH4 reacts with 4 mol of O2, how many moles of CO2 are formed? What is the limiting reactant? 2. How many grams of lead II chloride are produced from the reaction of 16.2 g of NaCl and 62.0 g of Pb(NO3)27 What is the limiting reactant? How much excess is left over?arrow_forwardg and lear X Your one-time code - escobedoa x G how do you cover a ml to liter -x + keAssignment/takeCovalentActivity.do?locator-assignment-take Use the References to access important values if needed for this question. According to the following reaction, how many moles of carbon dioxide will be formed upon the complete reaction of 31.9 grams of carbon (graphite) with excess oxygen gas? C(s) + O₂(g) → CO₂(g) mol carbon dioxide Submit Answer Q Search L Retry Entire Group Ws LPI 2 more group attempts remaining BAGNOdı hp ☆ 口 Previous OG 1 6:5 5/18/arrow_forwardSay you have a stock of 15% NaCl and need a reaction concentration of 1% in a total reaction volume of 25ul. How many ul of the 15% NaCl stock would you need to add to the reaction tube?arrow_forward
- A 103.0 mL aqueous solution of 43.0 mM COCI2 is combined with 594.0 mL of aqueous 74.0 mM K3PO4. If the limiting reagent is completed consumed during this reaction, how many grams of solid precipitate will be produced? Express your answer in units of grams using at least three significant figures.arrow_forwardUsing molarity to find solute mass and solution volume g 3/5 A chemist adds 105.0 mL of a 0.721M barium chloride (BaCl₂) solution to a reaction flask. Calculate the mass in grams of barium chloride the chemist has added to the flask. Round your answer to 3 significant digits. Ś Uyen Kha "...arrow_forward0.279 g of the hydrate CoCl2•nH2O is heated to produce 0.197 g of anhydrous CoCl2 (MM = 129.83 g/mol). We want to use this to determine n, how many waters of hydration were in the original sample, be the end of the questions that follow. How many grams of water were in the original 0.279 g of hydrate? How many grams of CoCl2 were present on the original 0.279 g of hydrate? So ... we now have (as a result of the previous two questions) what we need to know to determine the empirical formula of CoCl2•nH2O. We can look at CoCl2•nH2O in this way (CoCl2)x•nH2O. Recalling that x and n are whole number molar ratios ... use the results of the last two problems to determine the correct formula.arrow_forward
- A student was asked to calculate the mass of NaCl (in g) needed to create 500 g of 10 % NaCl solution. The student's work is below. Explain the student's mistake and identify what the correct answer should be. Given Looking for 500g I0% Na CI 9 Na Cl formula : %. = Solute in 9 x 100 Sdution in 9 Work (0 =X 500 X 100 10 X 10 = x 500 Answer = 0.2 g of Nacl lo0 500 0.2 %3D BI U Format ...arrow_forwardCan you please help me solve thisarrow_forwardHow much 20.0% NaOH should I put in a 30.0 ml tube if my dilution ratio is 1/10. What is the concentration of the tube. ml of 20.0% Just put the number of mL Do not enter anything but a number. Use correct sig figs. Remember ratios and conversion factors do not enter the significant figure calculation. What is the concentration in percent. Again, correct significant figures. NUMBERS only not the % signarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY