
Elements Of Electromagnetics
7th Edition
ISBN: 9780190698614
Author: Sadiku, Matthew N. O.
Publisher: Oxford University Press
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
A closed, rigid tank contains 4 kg of air initially at 300 K, 1 bar. As illustrated in the figure below, the tank is in contact with a thermal reservoir at 600 K and heat transfer occurs at the boundary where the temperature is 600 K. A stirring rod transfers W = -480 kJ of energy as shown in the diagram.The final temperature is 600 K. The air can be modeled as an ideal gas with cν = 0.733 kJ/kg · K and kinetic and potential energy effects are negligible.
Determine the amount of entropy transferred into the air and the amount of entropy produced, each in kJ/K.
| Q/Tb = | kJ/K |
| σ = | kJ/K |
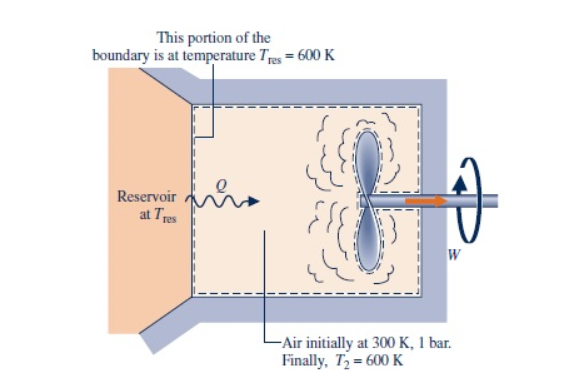
Transcribed Image Text:This portion of the
boundary is at temperature Tres 600 K
Reservoir
at Tres
W
- Air initially at 300 K, 1 bar.
Finally, T2 600 K
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 5 steps with 5 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Similar questions
- A rigid, insulated vessel is divided into two compartments connected by a valve. Initially, one compartment, occupying 1.0 ft³, contains air at 50 lbf/in², 675°R, and the other, occupying 2.0 ft³, is evacuated. The valve is opened and the air is allowed to fill both volumes. Assume the air behaves as an ideal gas and that the final state is in equilibrium. Determine the final temperature of the air, in °R, and the amount of entropy produced, in Btu/ºR. Step 1 Your answer is correct. Determine the final temperature of the air, in °R. Tf= 675 Hint °R Attempts: 1 of 4 usedarrow_forwardRefrigerant 134a at p1 = 30 lbf/in2, T1 = 40°F enters a compressor operating at steady state with a mass flow rate of 350 Ib/h and exits as saturated vapor at p2 = 16O Ib/in?. Heat transfer occurs from the compressor to its surroundings, which are at To = 40°F. Changes in kinetic and potential energy can be ignored. The power input to the compressor is 3.5 hp. Determine the heat transfer rate for the compressor, in Btu/hr, and the entropy production rate for the compressor, in Btu/hr.°R.arrow_forward2.3 0.2 m3 of a gas at 105 kPa and 27 0C is compressed until the temperature is 282 0C and the pressure is 1.25 MPa. If the specific heats of gas are Cv = 0.653 and Cp = 0.845 kJ/kg.K, determine,(a) Mass of gas(b) The volume at the end of compression(c) The change in internal energyarrow_forward
- Steam, initially at 5 MPa, 280°C undergoes a polytropic process in a piston–cylinder assembly to a final pressure of 20 MPa. If the polytropic constant, n = 1.4, calculate the non-flow work in kJ/kg.arrow_forwardCarbon dioxide (molar mass 44 kg/kmol) expands reversibly in a perfectly thermally insulated cylinder from 3.7 bar, 220 0C to a volume of 0.085 m3. If the initial volume occupied was 0.02 m3, calculate the adiabatic index to 1 decimal place. Assume nitrogen to be a perfect gas and take cv = 0.63 k J / k g Karrow_forwardCalculate the energy requirement to raise the temperature of 1 kg of water from 60 ° C of water to 90 ° C using the following approach; a. Average specific heat usage (Tab A Singh's Book. 4.1) = kJ b. The enthalpy change in the water - vapor saturation table (Tab A Singh's Book. 4.2) = kJarrow_forward
- Refrigerant 134a at p1 = 30 lb/in?, T1 = 40°F enters a compressor operating at steady state with a mass flow rate of 400 lb/h and exits as saturated vapor at p2 = 160 lb;/in?. Heat transfer occurs from the compressor to its surroundings, which are at To = 40°F. Changes in kinetic and potential energy can be ignored. The power input to the compressor is 4 hp. Determine the heat transfer rate for the compressor, in Btu/hr, and the entropy production rate for the compressor, in Btu/hr-°R.arrow_forwardRefrigerant 134a at p1 = 30 lbş/in?, T1 = 40°F enters a compressor operating at steady state with a mass flow rate of 250 lb/h and exits as saturated vapor at p2 = 160 lbę/in?. Heat transfer occurs from the compressor to its surroundings, which are at To = 40°F. Changes in kinetic and potential energy can be ignored. The power input to the compressor is 2.5 hp. Determine the heat transfer rate for the compressor, in Btu/hr, and the entropy production rate for the compressor, in Btu/hr-°R.arrow_forwardA piston–cylinder assembly contains 0.7 lb of air initially at a pressure of 30 lbf/in2 and a temperature of 400oF. The air is heated at constant pressure until its volume is doubled. Assume the ideal gas model with constant specific heat ratio, k = 1.4. Determine the work and heat transfer, in Btu.arrow_forward
- During a steady flow process, 4 kg of steam at 15 bar and |260 °C loses 3771 kJ of heat at constant pressure. Determine the final condition of steam.arrow_forwardFor each case, determine the specified property value and locate the state sketches of the p–υ and T–υ diagrams. For Refrigerant 134a at T = 160°F, h = 127.7 Btu/lb. Find υ, in ft3/lb. For Refrigerant 134a at T = 90°F, u = 72.71 Btu/lb. Find h, in Btu/lb. For ammonia at T = 160°F, p = 60 lbf/in.2 Find u, in Btu/lb. For ammonia at T = 0°F, p = 35 lbf/in.2 Find u, in Btu/lb. For Refrigerant 22 at p = 350 lbf/in.2, T = 350°F. Find u, in Btu/lb.arrow_forwardAs shown in the figure below, a gas contained within a piston–cylinder assembly, initially at a volume of 0.1 m3, undergoes a constant-pressure expansion at p = 3 bar to a final volume of V2 = 0.16 m3, while being slowly heated through the base. The change in internal energy of the gas is 0.25 kJ. The piston and cylinder walls are fabricated from heat-resistant material, and the piston moves smoothly in the cylinder. The local atmospheric pressure is 1 bar. (a) For the gas as the system, evaluate work and heat transfer, each in kJ.(b) For the piston as the system, evaluate work and change in potential energy, each in kJ.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY

Elements Of Electromagnetics
Mechanical Engineering
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:9780134319650
Author:Russell C. Hibbeler
Publisher:PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:9781259822674
Author:Yunus A. Cengel Dr., Michael A. Boles
Publisher:McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:9781118170519
Author:Norman S. Nise
Publisher:WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:9781337093347
Author:Barry J. Goodno, James M. Gere
Publisher:Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:9781118807330
Author:James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:WILEY