
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
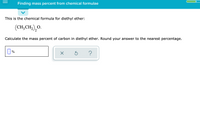
Transcribed Image Text:Finding mass percent from chemical formulae
This is the chemical formula for diethyl ether:
(CH,CH,),O.
О.
Calculate the mass percent of carbon in diethyl ether. Round your answer to the nearest percentage.
0%
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Imagine you discover a substance in a forest mushroom and find through experimentation that it efficiently kills viruses but is not toxic to animal cells. You analyze its elemental composition to be: 70.08% C, 11.04% H, and remainder O by mass. What is the empirical formula of this interesting substance? Show full calculations.arrow_forwardorganic solvent) that contains 1.00 x 1012 molecules 2. Calculate the mass of chloroform (CHC13, an of chloroform.arrow_forwardIs this correct answer?arrow_forward
- Which element has the largest mass % in alcohol CH3CH₂OH? (Hint: calculate the "mass" of each atom, C, H, O, in the compound) All three elements are the same! H Oarrow_forwardConvert 1.36 x 10-18 grams of X3Z5 into molecules/formula units of the same compound. (Assume that the molar mass of X is 32.36 grams per mole and the molar mass of Z is 56.03 grams per mole.) Assume that Avogadro's number is 6.022 x 1023. Report your answer to three decimal places.arrow_forwardHow many atoms of chloride are in 2.38 moles of sodium chloride? I have the answer as 1.43 x 10^24. But i am so lost on how to get to that answer, i need a step by step solution on how to find it. Thank you :)arrow_forward
- One form of asbestos called chrysotile is considered to be a human carcinogen. Mass analysis shows that the composition of chrysotile is 26.3% Mg, 20.2% Si, 1.45% H, and the remainder of the mass is oxygen. Determine the empirical formula of chrysotile. ( Type in the formula without spaces or subscripts in the order it appears in the problem. As in Mg1Si2H3O4)arrow_forwardHow to i measure the mass oxalate when i only have the potassium ferrioxalate sample of 0.127 g but no grams of oxalate? please helparrow_forwardA 3.000 g sample of a gaseous compound was found to contain 2.560 g of carbon and 0.440 g of hydrogen. A) What is the simplest formula of the compound (Write C first and then H)? You won't be able to make the numbers a subscript. For instance, for MgCl2 enter MgCl2. B) The molar mass of the compound was found to be 42.08 g/mol. What is the molecular formula of the compound? You won't be able to make the numbers a subscript. For instance, for MgCl2 enter MgCl2.arrow_forward
- This is the chemical formula for acetone: (CH3) CO. Calculate the mass percent of carbon in acetone. Round your answer to the nearest percentage. 1% X Śarrow_forwardThis is the chemical formula for methyl acetate: (CH3)₂ CO₂. Calculate the mass percent of carbon in methyl acetate. Round your answer to the nearest percentage. %arrow_forward20) Write the empirical formulas for the following compound: HH -0- A) C,H¿O2 B) CH,0 C) CH,O 21) Write a sentence that describes how to determine the number of moles of a compound in a known mass of the compound if we know its molecular formula. A) Use the molecular formula to find the molar mass; to obtain the number of moles, divide the molar mass of the compound by the mass of compound. B) Use the molecular formula to find the molar mass; to obtain the number of moles, divide the mass of compound by the molar mass of the compound. C) Use the molecular formula to find the molar mass; to obtain the number of moles, multiply the mass of compound with the molar mass of the compound. 22) Compare 1 mole of H, 1 mole of O, and 1 mole of F2. Which has the largest number of molecules? Which has the greatest mass? A) All three have the same number of molecules, since a mole is defined as a specific number of particles. B) O2 has the largest number of molecules. C) H2 has the largest number…arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY