
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
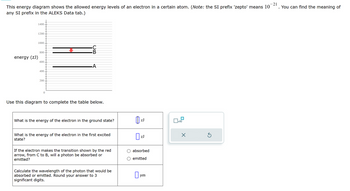
Transcribed Image Text:This energy diagram shows the allowed energy levels of an electron in a certain atom. (Note: the SI prefix 'zepto' means 10
any SI prefix in the ALEKS Data tab.)
energy (ZJ)
1400
1200
1000
800
600
A
400
200
0
Use this diagram to complete the table below.
What is the energy of the electron in the ground state?
ZJ
☐ x10
☑
What is the energy of the electron in the first excited
state?
If the electron makes the transition shown by the red
arrow, from C to B, will a photon be absorbed or
emitted?
Calculate the wavelength of the photon that would be
absorbed or emitted. Round your answer to 3
significant digits.
☐ ZJ
absorbed
emitted
☐
um
21
. You can find the meaning of
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 1 steps with 1 images

Knowledge Booster
Similar questions
- of 33 > % A In scenario C, visible light is in the middle of the yellow region of the visible spectrum. Estimate its wavelength, frequency, and energy per photon. frequency: In scenario D, visible light has a photon energy of 4.151 x 10-19 J. Determine its wavelength, frequency, and color. frequency: O a R LE 5 25 6 00 G H 8 ST1 $1 9 K wavelength: energy per photon: wavelength: The visible light in scenario D is RA L P 90°F nm nm J 3:36 PM 7/28/2022arrow_forward> Question Completion Status: QUESTION 4 Roaches for sale | C... For Sale/Trade/Wan... * Tarantulas CSL Behring Hell Let Loose TR St... Indicate which three of the following statements about the electron in a hydrogen atom are true by checking the box in front of each true statement. The atom is in the ground state when the electron is in the n=1 level. Energy is released when the electron is excited from the n=1 level to the n=3 level. A shorter wavelength of light is emitted when the electron moves from the n=3 level to the n=2 level than when the electron moves from the n=4 level to the n=2 level. Energy is always released when an electron relaxes. A lower frequency of light is emitted when the electron moves from the n=3 level to the n=2 level than when the electron moves from the n=4 level to the n=2 level. The atom is stable when the electron relaxes to the n=2 level.arrow_forwardneed it fast! In a hydrogen atom, an electron loses energy from n = 5 to n = 2, a process that leads to some kind of “interaction” with electromagnetic radiation. What is the energy of this electromagnetic radiation in Joules? Do NOT include a sign with this answer. Rydberg's constant is equal to -2.179 x 10-18 J. Express exponents as number followed by E followed by the power (e.g., 7.34E-12).arrow_forward
- A hydrogen atom absorbs a photon of ultraviolet light, exciting an electron from energy level 1 to energy electron drops back down in three steps, as shown. Using the table, identify the energy released in each tr visible, or infrared. 7 Transition from 7→5: 6. E = 4.23 x 10-20 J 2 = 4,668 nm 4 Transition from 5→ 2: E = 4.58 × 10-19 J 1 = 434 nm 2 Transition from 2→ 1: E = 1.63 x 10-18 J 1 = 122 nm Transition Wavelength Region 7 → 5 4,668 nm 5 → 2 434 nm 2 → 1 122 nm Answer Bank ultraviolet infrared visible Energyarrow_forwardConsider the four scenarios involving visible light. In scenario A, visible light has a wavelength of 729.9 nm.729.9 nm. Determine its frequency, energy per photon, and color. frequency: s−1−1 energy per photon: J The visible light in scenario A is In scenario B, visible light has a frequency of 5.490×1014 s−1.5.490×1014 s−1. Determine its wavelength, energy per photon, and color. wavelength: nm energy per photon: J The visible light in scenario B is In scenario C, visible light is yellow. Determine its wavelength, frequency, and energy per photon. wavelength: nm frequency: s−1−1 energy per photon: J In scenario D, visible light has a photon energy of 4.202×10−19 J.4.202×10−19 J. Determine its wavelength, frequency, and color. wavelength: nm frequency: s−1−1 The visible light in…arrow_forwardWhat is the formal charge of carbon in the following resonance structure of CH-2N2? (Can you draw the other resonance forms?)arrow_forward
- Consider a hydrogen atom in the ground state. What is the energy of its electron? E = Now consider an excited-state hydrogen atom. What is the energy of the electron in the n = 3 level? E3 = PAGES · O J Jarrow_forwardledu.co Question 16 of 16 In Einstein's photoelectric experiment, the kinetic energy (K.E.) of an electron displaced from a metal by a photon was the difference between the energy of a photon and a threshold energy value for the metal. If you were to perform a similar experiment with lead, which has a threshold energy of 6.81 x 10 19 J, and ultraviolet light of wavelength 38.5 nm, what would be the speed (v) of the dislodged electron? (K.E. = 1/2mv2. The mass of the electron = 9.109 x 10 1 kg.) | m/s 9. 4 8. x 100 +/- A ENG to search 60arrow_forwardAn unidentified element is known to have an electron configuration, [X] ns2, in its ground state. This element must be in the same family as radon (Rn) O rubidium (Rb) arsenic O radium (Ra) O leadarrow_forward
- 4. Electrons must gain energy in order to move from lower energy levels to higher energy levels. When the electron moves back down from a higher energy level to a lower one, how does it "lose" this energy? Where does the energy go? Edit View Insert Format Tools Table 12pt v Paragraph v BIU T?v:arrow_forwardAn atom emits light at various wavelengths, two of which are 434 nm and 472 nm. Both of these transitions are to the same final state. What is the energy difference between the two states for each transition ? 3.7 x 10 - 20 J O 1.2 x 10 - 19 O 4.2 x 10- 20J O 3.4 x 10 - 19arrow_forwardany SI prefix in the ALEKS Data tab.) 1400 - 1200- 1000 800 energy (z) 600 400 200 Use this diagram to complete the table below. What is the energy of the electron in the ground state? What is the energy of the electron in the first excited state? If the electron makes the transition shown by the red arrow, from C to A, will a photon be absorbed or absorbed emitted emitted? Calculate the wavelength of the photon that would be absorbed or emitted. Round your answer to 3 significant digits. nm I Don't Know Submit © 2022 McGraw Hill LLC. All Righarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY