
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
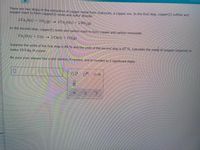
Transcribed Image Text:There are two steps in the extraction of copper metal from chalcocite, a copper ore, In the first step, copper(1) sulride and
oxygen react to form copper(1) oxide and sulfur dioxide:
2 Cu,S(3) + 30,(g)
2 Cu,O(s) + 2 SO,(8)
In the second step, copper(1) oxide and carbon react to form copper and carbon monoxide:
CusO(s) + C(s)
2 Cu(s) + CO(g)
Suppose the yield of the first step is 86.% and the yield of the second step is 67.%. Calculate the mass of oxygen required to
make 10,0 kg of copper.
Be sure your answer has a unit symbol, if needed, and is rounded to 2 significant digits.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The compound sodium thiosulfate pentahydrate, Na2S2 O3-5H2O, is important commercially to the photography business as "hypo," because it has the ability to dissolve unreacted silver salts from photographic film during development. Sodium thiosulfate pentahydrate can be produced by boiling elemental sulfur in an aqueous solution of sodium sulfite. S8 (s) + Na2 SO3 (aq) + H2O(1) → Na2 S2 O3-5H2 O(s) (unbalanced) What is the theoretical yield of sodium thiosulfate pentahydrate when 3.25 g of sulfur is boiled with 13.1 g of sodium sulfite? Sodium thiosulfate pentahydrate is very soluble in water. What is the percent yield of the synthesis if a student doing this experiment is able to isolate (collect) only 5.26 g of the product?arrow_forwardStandard airbags for automobiles used to implement the formation of sodium azide as a source of nitrogen gas. This has since been replaced due to the potential caustic nature of the products. The sodium azide required for automobile air bags is made by the reaction of sodium metal with dinitrogen oxide in liquid ammonia: 3N2O(g) + 4Na(s) + NH3(l)→ NaN3(s) + 3NaOH(s) + 2N2(g) a. You have 65.0 g of sodium and a 35.0 L flask containing N2O gas with a pressure of 2.12 atm at 296 K. What is the maximum possible yield (in grams) of the NaN3?arrow_forwardBalance the cquation C,H18() + 0>(g)→ CO,(g) + H;O(g) CO,(g) + H,0(g)arrow_forward
- There are two steps in the extraction of copper metal from chalcocite, a copper ore. In the first step, copper(I) sulfide and oxygen react to form copper(I) oxide and sulfur dioxide: →+2Cu2Ss 3O2g + 2Cu2Os 2SO2g In the second step, copper(I) oxide and carbon react to form copper and carbon monoxide: →+Cu2Os Cs + 2Cus COg Suppose the yield of the first step is 86.% and the yield of the second step is 64.% . Calculate the mass of copper(I) sulfide required to make 2.0kg of copper. Be sure your answer has a unit symbol, if needed, and is rounded to the correct number of significant digits.arrow_forwardIn a blast furnace, coke, which is solid carbon, reacts with O2(g) to form carbon monoxide. The carbon monoxide then reacts with Fe2O3(s) to produce solid iron and carbon dioxide. Which of the following is the balanced equation for this combined process? O2 Fe2O3(s) + 3 C(s) + 3 02(g)->2 Fe(s) + 3 CO2(g) O2 Fe2O3(s) + 2 C(s) + 3 02(g)-> 4 Fe(s) + 2 CO2(g) O2 Fe2O3(s) + 6 C(s) + 3 02(g)--> 4 Fe(s) + 6 CO2(g) O2 Fe2O3(s) + C(s) + 3 O2(g) -> Fe(s) + CO2(g)arrow_forwardNitric acid can be formed in two steps from the atmospheric gases nitrogen and oxygen, plus hydrogen prepared by reforming natural gas. In the first step, nitrogen and hydrogen react to form ammonia: N,(0) + 3 H,(9) → 2 NH,(9) ΔΗ-92. k In the second step, ammonia and oxygen react to form nitric acid and water: NH,(9) + 20,(9) → HNO3(g) + H,O(g) AH=-330. kJ Calculate the net change in enthalpy for the formation of one mole of nitric acid from nitrogen, hydrogen and oxygen from these reactions. Round your answer to the nearest kJ. ?arrow_forward
- 2+ Natural waters often contain relatively high levels of calcium ion, Ca²+, and hydrogen carbonate ion (bicarbonate), HCO3¯, from the leaching of minerals into the water. When such water is used commercially or in the home, heating of the water leads to the formation of solid calcium carbonate, CaCO3, which forms a deposit ("scale") on the interior of boilers, pipes, and other plumbing fixtures. Ca(HCO3)2 (aq) → CaCO3 (s) + CO2(g) + H₂O(1) If a sample of well water contains 0.0016 mg of Ca(HCO3)2 per milliliter, what mass of CaCO3 scale would 1.0 mL of this water be capable of depositing? Mass= garrow_forwardThe manufacture of nitrogen trifluoride is a growing industry, due to its use as a plasma etchant in the semiconductor industry. Nitrogen trifluoride can be synthesized by passing a current through a high-temperature mixture of ammonium fluoride and hydrogen fluoride, with hydrogen gas as a byproduct. In one synthesis, 1.20x10^3g ammonium fluoride is reacted with1.20x10^3g hydrogen fluoride to produce 5.00x10^2g nitrogen trifluoride. What is the limiting reactant, excess reactant, theoretical yield of nitrogen trifluoride, percent yield, and mass of excess reactant remaining after the reaction?arrow_forwardThe reduction of iron(III) oxide to iron during steel-making can be summarized by this sequence of reactions: 20(s) +0, (g) =2 CO (g) K, Fe,0, (s) +3 CO (g) =2 Fe (1) +3 CO, (g) K2 The net reaction is: 2 Fe,O, (s) +6C (s) +30, (g)=4 Fe (1) +6CO, (3) K Write an equation that gives the overall equilibrium constant K in terms of the equilibrium constants K, and K,. If you need to include any physical constants, be sure you use their standard symbols, which you'll find in the ALEKS Calculator. K = 믐 Submit Assign Continue MacBook Air F7 000 吕口 000 FA F5arrow_forward
- Solid copper can be produced by passing gaseous ammonia over solid copper (II) oxide at high temperatures, according to the following reaction. NH3 (g) + CuO (s) → N2 (g) + Cu (s) + H2O (g) Balance the reaction.arrow_forwardWhat mass of Cu(IO3)2 can be formed from 0.650 g of CuSO4 · 5H2O? What mass of KIO3 is needed to convert the copper in 0.2750 g of CUSO4 - 5H2O to Cu(IO3)2?arrow_forwardBalance the chemical equation below using the smallest possible whole number stoichiometric coefficients.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY