
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%

Transcribed Image Text:Chemical reactions in the real world don't always go exactly as planned on paper. In the course of an experiment, many things
will contribute to the formation of less product than would be predicted. Besides spills and other experimental errors, there are
usually losses due to an incomplete reaction and undesirable side reactions. Chemists need a measurement that indicates how
successful a reaction has been. This measurement is called the percent yield.
To compute the percent yield, it is first necessary to determine how much of the product should be formed based on
stoichiometry. This is called the theoretical yield, the maximum amount of product that could be formed from the given
amounts of reactants. The actual yield is the amount of product that is actually formed when the reaction is carried out in the
laboratory. The percent yield is the ratio of the actual yield to the theoretical yield, expressed as a percentage.
Percent yield is very important in the manufacture of products. Much time and money is spent improving the percent yield for
chemical production. When complex chemicals are synthesized by many different reactions, one step with a low percent yield
can quickly cause a large waste of reactants and unnecessary expense.
Typically, percent yields are understandably less than 100 % because of the reasons indicated earlier. However, percent yields
greater than 100% are possible if the measured product of the reaction contains impurities that cause its mass to be greater
than it actually would be if the product was pure. When a chemist synthesizes a desired chemical, he or she is always careful to
purify the products of the reaction.
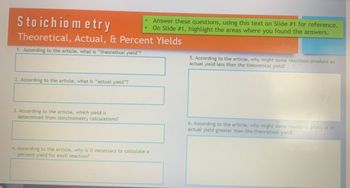
Transcribed Image Text:Stoichiometry
Theoretical, Actual, & Percent Yields
1. According to the article, what is "theoretical yield"?
2. According to the article, what is "actual yield"?
3. According to the article, which yield is
determined from stoichiometry calculations?
●
4. According to the article, why is it necessary to calculate a
percent yield for each reaction?
Answer these questions, using this text on Slide #1 for reference.
On Slide #1, highlight the areas where you found the answers.
5. According to the article, why might some reactions produce an
actual yield less than the theoretical yield?
6. According to the article, why might some reactions produce an
actual yield greater than the theoretical yield?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 5 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- someone help with this question!arrow_forwardTwo experimental procedures have the same reactants and the same products. Procedure A has 4 steps between reactants and products. Procedure B has 2 steps. Which procedure is likely to have a higher % yield and why? Be detailed and write in complete sentences.arrow_forwardQUESTION 23 Which of the following statements is false? The limiting reactant is completely consumed in a chemical reaction. O The theoretical yield is the amount of product that can be made based on the amount of limiting reagent. O The actual yield is the amount of product actually produced by a chemical reaction. O The percent yield = (actual yield/theoretical yield) x 100% O All of the above are true statements.arrow_forward
- Aspirin can be synthesized in the lab by combining salicylic acid (C,H,0,) and acetic anhydride (C,H,0,) to form aspirin ( C,H,O,) and acetic acid (C,H,O,). The balanced equation for this reaction is C,H,O, + C,H,O, → C,H,0, + C,H,O, A student started with 3.33 mL acetic anhydride (density = 1.08 g/mL) and 2.61 g salicylic acid. The student synthesized 2.53 g of aspirin. Select the limiting reactant. salicylic acid (C, H,0,) acetic anhydride (C,H,O;) aspirin (C,H,O,) O acetic acid (C,H,O,) Calculate the theoretical yield of aspirin (C,H,O,). theoretical yield: Calculate the percent yield for aspirin (C,H,O).arrow_forwardDo not give handwriting solution. Determine the percent yield to two decimal places if 5.53 g of alum are synthesized using 0.43 g of aluminum. (MM Al = 26.98 g/mol; MM alum = 474.38 g/mol)arrow_forward* Question Completion Status: A Moving to another question will save this response. Question 25 "How many grams of carbon dioxide will be produced by the complete combustion of 50 grams of C 5H 12? With 128.62grams of oxygen? Enter the coefficients for the balanced chemical equation (enter "" 1" for compounds without a coefficient) C5H 12+ 02 - H20 How many grams of carbon dioxide are produced from the amount of hydrocarbon? round your answer to the nearest whole number How many grams of carbon dioxide are produced from the amount ofoxygen? round your answer to the nearest whole number Which is reactant is the limiting reactant? type ""oxygen"" or ""hydrocarbon"" How many grams of carbon dioxide is produced? round your answer to the nearest whole number A Moving to another question will save this response.arrow_forward
- Iron reacts with oxygen at high temperatures to form iron(III) oxide. 4 Fe(s) + 30,(g) 2 Fe,0,(s) Suppose 17.2 g of iron (Fe) is reacted with 16.6 g of oxygen (0,). Select the limiting reagent. O2 Fe O Fe,O, Calculate the theoretical yield of iron(II) oxide (Fe, O,). 3 aldarrow_forwardThe combustion of methane produces carbon dioxide and water. Assume that 52.8 grams of methane burned in the presence of excess oxygen gas. The percent yield is __________ percent if the reaction produces 100.9 grams of carbon dioxide.arrow_forward1) The Law of Conservation of Mass states that the total mass, in an open system, does not change as the result of reactions between its parts. True False 2) The deviation from the theoretical yield to the actual yield is called the percent yield. True Falsearrow_forward
- Hi! I need help with these limiting reactants questions from my Chem class pleasearrow_forwardPlease correct answer and don't use hand ratingarrow_forwardVisited Use the References to access important values if needed for this question. According to the following reaction, how many grams of iron(III) chloride will be formed upon the complete reaction of 22.1 grams of chlorine gas with excess iron? 2Fe(s) + 3Cl₂(g) → 2FeClę (s) giron(III) chloride Submit Answer Q Search H N & 81 U 18 * M Retry Entire Group 29. • C fo K hp 9 more group attempts remaining no 111 O 12-->> [ ** ins W prt sc. Previous (NEW) delete backspace home lock enter 1 o 5:35 PM 5/14/2023 end 7 Varrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY