
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
which one is the highest boiling point?
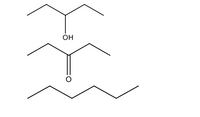
Transcribed Image Text:OH
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemical-engineering and related others by exploring similar questions and additional content below.Similar questions
- -67 Verify the temperature distribution for case 2 in Section 2-9, i.e., that T – T cosh m(L – x) +(h/mk) sinh m(L – x) То — То cosh mL+ (h/mk) sinh m L Subsequently show that the heat transfer is sinh mL+ (h/mk) cosh m L cosh mL+ (h/mk) sinh mL q=/h PkA (To – T∞) that of the surrounding fluid. CASE 2 The fin is of finite length and loses heat by convection from its end. If we let m-h P/kA, the general solution for Equation (2-306) may be written 6=Cje +C 12-31] For case I the boundary conditions are e = at x=0 at x00 CHAPTER 2 Steady-Sute Conduction One Dimension and the solution becomes e T-Tx 12-32] To -T For case 3 the boundary conditions are e = at x0 de -0 at x=L dx Thus C +C2 0mm(-Cjemt +Czey Solving for the constants C, and C2, we obtain + 12-33a] cosh [m(L-x)1 12-336| cosh ml. The hyperbolic functions are defined as e +e 2 sinh x cosh x sinh x - - cosh x tanh x= e te The solution for case 2 is more involved alecbraically and the result is T-T _cosh m (L -x) + (h/mk) sinh m (L…arrow_forwardPlease answer the whole question 3 a - b - varrow_forwardSolve step by step Solution is provided!arrow_forward
- What are the relative humidity and dew point temperature of 1 kilogram of 25 degree Celsius air that contains 10 grams of water vapor?arrow_forwardEstimate the heat rate that must be supplied to vaporize 100 moles per hour of n-hexane at 25°C and 7 bar to 300°C at constant pressure.arrow_forward17D.2. When a sample of gold iscooled from 32.5 °C to 20.0 °C,9.46 J of heat are liberated. What isthe mass of the sample if the specificheat of gold is 0.129 J/g·°C?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The