
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
The bar graph shows the degree of ionization of 1 × 10–3 M solutions of three hypohalous acids: HOCl, HOBr, and HOI.
Which bar is the one for HOI?
Choose one:
a. The tallest bar.
b. The middle bar.
c. The shortest bar.
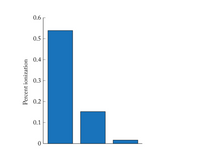
Transcribed Image Text:0.6
0.5
0.4
0.3
0.2
0.1
Percent ionization
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Determine the pH of a 0.588 M weak base soultions at 25C. The Kb for the weak base is 1.76 x 10^-5. a. 9.25 b. 2.49 c. 4.75 d. 11.34 e. 11.51arrow_forwardFor numbers A to E, each of the following items concerns a 0.10 M solution of a weak organic base, B. Identify whether the statements are true or false. Shade A if the statement is correct and accurate; if otherwise, shade B. A. OH-]equals 10 M. B. [B]is much lesser than [HB+]. C. [H3O+]is greater than [HB+]. D. ThepH is E. [HB+]is approximately equal to [OH-]. For numbers F to J, consider the given case: Blood contains several acid-base systems that tend to keep its pH constant at about 7.40. One of the most important buffer systems involves Carbonic acid and Bicarbonate ion. Ka=4.4 x 10-7. F. In the carbonic acid-bicarbonate ion buffersystem, which of the following functions as a weak acid? H3CO3+ H2CO3 HCO3- CO32- CO2 G. In the carbonic acid-bicarbonate ion buffersystem, which of the following functions as a conjugate base? H3CO3+ H2CO3 HCO3- CO32-CO2 H. Whatmust be the ratio of bicarbonate to carbonic acid in the blood if the pH is 7.40? 11.05:1 2.95:2…arrow_forward29. Consider the solutions in question 28. This solution has the lowest pH. A. HCI В. NaOH C. NH3 D. Acetic acid E. NaCIarrow_forward
- which two solutions have the lowest concentration of H+ ions? 1. window cleaner 2. detergent 3. HCl 4. vinegar 5. baking soda 6. lemon juice 7. drano 8. NaOHarrow_forwardDescribe each of the following solutions as basic, acidic, or neutral.arrow_forwardIndicate if the following solutions are acidic or basic. Consider 25 °C. а. рH%3D2.50 b. [H*]> 1.0 x 10-7 с. РОН 3D 3.28 d. [OH]= 2.11 × 10-12 Marrow_forward
- The pH of a 0.92M solution of hypobromous acid (HBRO) is measured to be 4.34. Calculate the acid dissociation constant K, of hypobromous acid. Round your answer to 2 significant digits. K %3D a x10 Canti 2021 McGraw Hill LLC.arrow_forwardIndicate whether or not both members of each of the following pairs of substances are salts. a. LiOH and LiBr b. NaClO and HCIO c. KF and K,O d. K.S and K,SO,arrow_forwardaring Part A For 250.0 mL of pure water, calculate the initial pH and the final pH after adding 0.031 mol of NaOH. Express your answers to two decimal places. Enter your answers numerically separated by a comma. 15. ΑΣΦ pHinitial. PHfinal= B ? gaarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY